Dangsheng Su, Jian Zhang, Rui Wang at Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), Robert Schlögl at Fritz Haber Institute, Xiangguang Yang at Changchun Institute of Applied Chemistry (CAS), Andreja Gajovic at Rudjer Boskovic Institute (Croatia), and co-workers realized the steam- and coke-free dehydrogenation of ethylbenzene by using the nanodiamond as the catalyst. With the absence of gaseous oxygen or steam, the nanodiamond displayed the activity three times of the commercial iron oxide catalyst. After a long-term of reaction, the surface of nanodiamond is free of deposited carbon. This new strategy demonstrates a promising future in the ethylbenzene dehydrogenation industry. The full text is available on the Angewandte Chemie International Edition journal. Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201002869/abstract
Basic research of catalysis science has essentially related with the development of modern chemical industries. Around 80% chemicals are produced directly or indirectly by the catalytic processes. Strict requirements for a high-performance catalyst include a long-term activity, high thermal conductivity, good mechanical properties and recyclability. For the direct dehydrogenation of alkanes, conventional catalysts are the supported metals or their oxides nanoparticles. Along with the activation process, the dissociation of alkane molecules inevitably happens to form carbon deposits on the active sites. This will reduce the surface area, porosity and number of active sites, resulting in a sharp loss of activity of the catalysts. Such a "coking" phenomenon has strongly restricted the progress of alkane conversion. The present anti-coking strategy is to introduce a large amount of steam as the protection agent, causing the huge energy consumption. With the depletion of fossil fuels and worsening global climate, it is necessary to develop a new energy-saving, coke-free and efficient catalyst for alkane dehydrogenation.
Carbon nanomaterials have been usually produced via the severe chemical processes such as chemical vapor deposition, arc discharge, laser ablation, etc. Thus a large amount of structural defects were introduced to the surface of commercial nanocarbons. Curving of graphitic structure in the scale of several nanometers will further delocalize the free electrons of graphite domains, featuring an enhanced chemical reactivity of carbon atoms. After the simple functionalization, the surface of nanocarbon will be rich of oxygen- or nitrogen-containing groups with some acid/base properties and reduction/oxidation ability.
In this study, it is found that the nanodiamond nanoparticle is in a diamond-graphene core-shell structure. The surface graphene layer is highly defective and rich of oxygen-containing groups, among which the ketone-like C=O species can be stable even at temperature as high as 550oC. To elucidate the reaction mechanism, the researchers have applied in-situ synchrotron-excited XPS and diffuse reflectance infrared spectroscopy to follow the change in chemical bonding under the reaction conditions. The results have well explained the structure of active sites, transient state of adsorbed ethylbenzene, and metal-free reaction turnover.
Other related work from the researchers about the metal-free carbon catalysis can be found in the series of publications on Science (2009, 322, 73), Angew. Chem. Int. Ed. (2007, 46, 7319; 2009, 48, 6913), J. Am. Chem. Soc. (2009, 131, 11296), Advanced Materials (2008, 20, 1450; 2009, 22, 87), Chemical Communications (2007, 1916; 2008, 6528), ChemSusChem, etc.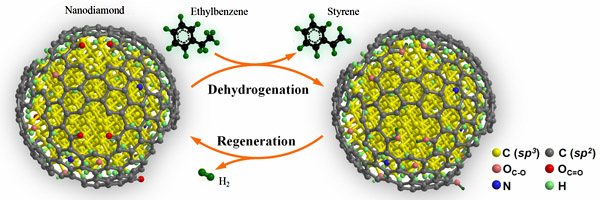
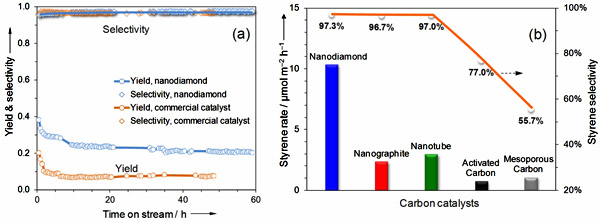
Figure 2 Results of catalytic evaluations and in-situ characterization of nanodiamond. (a) Steam-free dehydrogenation of ethylbenzene at 550oC. Conditions: 50 mg catalyst, 2.8% ethylbenzene in helium, gas flow rate 10 mL/min, 1 atm. (b) In-situ synchrotron-excited XPS spectra of nanodiamond under vacuum and ethylbenzene vapor at 400-450oC.