Single-walled carbon nanotubes (SWCNTs) are recognized as a landmark material in nanoscience and nanotechnology. SWCNT can be imagined to be a seamless tube rolled up from a single layer graphene sheet. A SWCNT can be either metal or semiconductor, depending on its chirality index. In addition, the energy band gap of semiconducting SWCNT is inversely proportional to its diameter. SWCNTs can be produced by arc discharge, laser ablation, and chemical vapor deposition (CVD) techniques. However, current methods can only grow SWCNTs as a mixture of various diameters, lengths, and electronic properties, since precise understanding on their growth mechanism and proper controlled growth method have not been achieved.
Iron-group metals have long been regarded as the only suitable catalyst for SWCNT growth due to their abilities for catalytic dissociation of carbon-bearing molecules, suitable carbon solubility, and the capability for graphitilization of carbon. Previous studies revealed that catalyst is a key factor that determines the structure of the SWCNTs synthesized, and various new metals have been developed for SWCNT growth since 2006. However, the metal residual in SWCNTs cannot be removed completely, which may influence the intrinsic properties of SWCNTs and hinders their practical applications in electronics and biomedical fields.
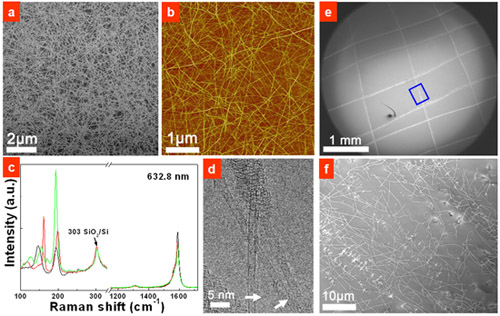
Recently, researchers from Shenyang National Laboratory for Materials Science (SYNL) have devised a simple and efficient approach to realized metal-catalyst-free growth of high quality SWCNTs. They first sputtered 30-nm-thick SiO2 film on silica wafers and found that SiOx nanoparitcles (NPs) with an average size of 1.9 nm formed after a heat treatment under H2. After CH4 CVD at 900 °C, dense, clean, and pure SWCNT were obtained on the wafer (Figure 1a-d). In addition, they have also developed a simple “scratching growth” approach for the metal-free and patterned growth of SWCNTs (Figure 1e, f). This work was recognized as “A Surprising Discovery”(Andreas Hirsch, Angew. Chem. Int. Ed. 2009, 48, 5403).
Further studies on the growth of SWCNTs from SiOx catalyst found that SWCNTs are grown on SiOx at a very low rate of 8.3 nm/s, 300 times slower than that of commonly used Co catalyst. Via cooperation with the researchers from the University of Queensland in Australia, they have shown, from density functional theory (DFT) calculations, that the slow grow rate of SWCNTs stems from the slow decomposition of CH4 and the reversible adsorption of CH4 on silica surface. The slow growth rate of SWCNTs on SiOx renders the directly length-controlled synthesis of short SWCNTs with an average length of only 149 nm.
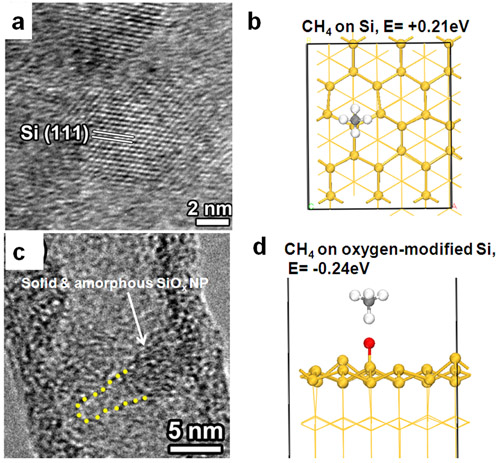
The nature of catalyst is critical to understand SWCNT growth mechanism. Recently, by using in situ TEM, CVD, and DFT calculations, these researchers have unveiled the real catalyst functions is solid and amorphous SiOx NPs. Consequently, SWCNTs growth follows a vapor-solid-solid mechanism, instead of the traditional vapor-liquid-solid mechanism. Further in situ TEM and CVD studies found that Si NPs cannot grow SWCNTs, indicating that the chemical composition of the catalyst is a key factor for SWCNT growth. DFT calculations revealed that the oxygen in SiOx enhances the adsorption of CH4 on catalyst and facilitates SWCNTs growth on the oxygen-containing silica NPs. The above results provide important implications for exploring and designing new catalysts for the efficient and controllable growth of SWCNTs.
Some of the above results were published in J. Am. Chem. Soc. (2009, 131, 2082; 2011, 133, 197) and ACS Nano (2009, 3, 3421).