Supported metallic nanoparticles (NPs) with their potential in a wide range of current and future applications is one of the fastest growing research fields. As the indispensable component, catalyst support has an essential influence on the supported catalyst, as it can influence the dispersion and morphology of metallic nanoparticles. Besides that, the interaction between the metallic particles and the support can affect the catalytic performance. Therefore, the search for novel support materials with exceptional structures and properties that enhance the catalytic performance of metal nanoparticles (NPs) remains an important topic in heterogeneous catalysis.
Recently, Prof. SU Dangsheng, Associated Prof. LIU Hongyang and PhD student ZHANG Liyun from Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Scieneces have fabricated a nanodiamond–graphene material comprising a nanodiamond core and a graphitic shell with just one or two graphene layers (ND@G) and used it to support Pd NPs. Compared to the traditional sp2-bonded onion-like carbon (OLC), ND@G supported Pd catalyst had an improved catalytic activity for CO oxidation reaction (Figure 1). High-angle angular dark field scanning transmission electron microscopy (HAADFSTEM), Pd K-edge X-ray absorption spectroscopy, and temperature-programmed CO desorption (CO TPD) measurements were used to study the mechanism. Compared with OLC, ND@G is composed of a sp3-bonded ND core and a sp2-bonded defective graphitic shell, which enhance the metal-support interaction in Pd/ND@G, and further modify the morphology and structure of Pd nanoparticles (Figure 2). And the sintering resistance of the Pd nanoparticles on ND@G were enhanced significantly (Figure 3). The strong metal-support interaction in Pd/ND@G weaken the CO chemisorption on Pd, and enhanced the chemisorption and dissociation of O2 on Pd, which improve the CO oxidation catalytic activity. This work was published as a communication on the journal of Angewandte Chemie International Edition (DOI: 10.1002/anie.201507821). http://onlinelibrary.wiley.com/doi/10.1002/anie.201507821/full
Several achievements have been made by this group to improve catalytic performances of the supported metallic catalyst by regulating the structure and properties of nanocarbon materials. Papers have been published in several international journals including Angewandte Chemie International Edition (2014, 53, 12634-12638), ChemCatChem (2014, 6, 2600-2606), Small (2015, 11, 5059-5064), Catalysis Today (2016, 260, 55–59).
These works are supported by MOST, NSFC, “Strategic Priority Research Program” of the Chinese Academy of Sciences, and Shanghai Synchrotron Radiation facility.
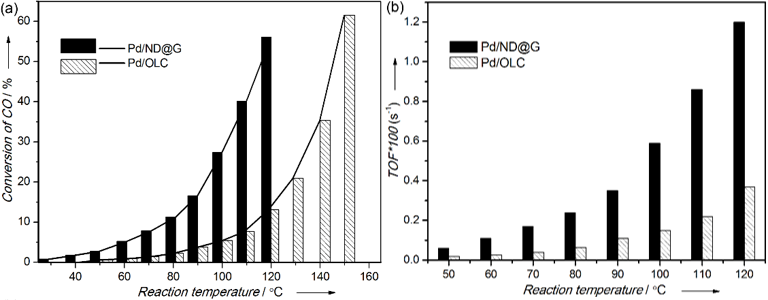
Figure 1. (a) Temperature dependence of the CO oxidation reaction for
the Pd/C catalysts. (b) TOF versus temperature for Pd/ND@G and Pd/
OLC samples (CO/O2=1:0.5)
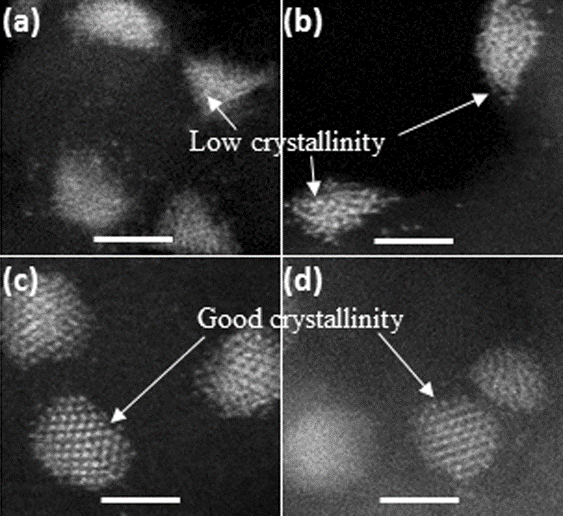
Figure 2. a–d) High-resolution HAADF-STEM images of Pd/ND@G
(a,b) and Pd/OLC (c, d). Scale bars: 2 nm
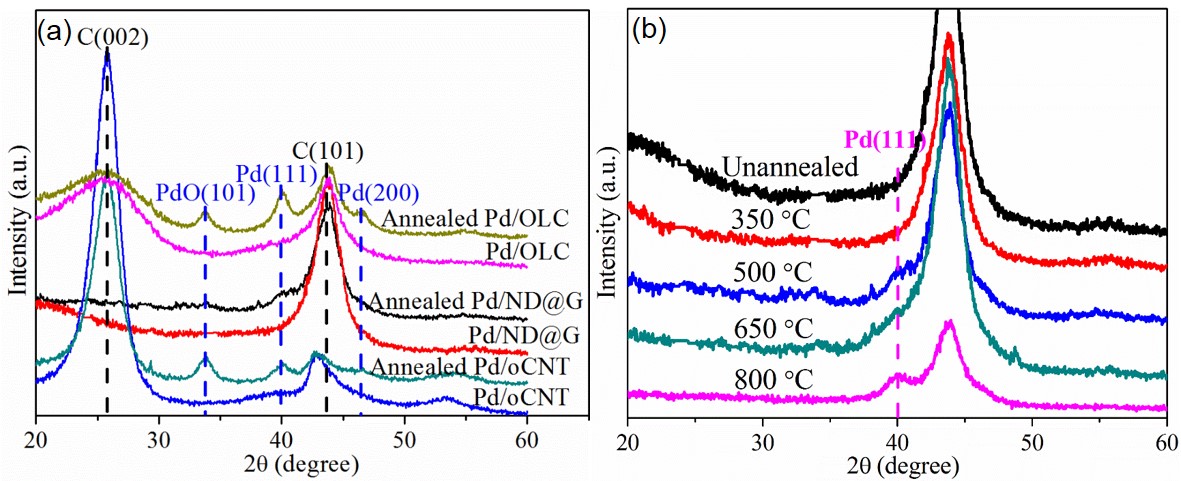
Figure 3. (a) XRD patterns of supported catalysts (Pd/ND@G, Pd/OLC and Pd/oOLC) fresh and annealed in Ar at 500 °C for 6 h and (b) XRD patterns of Pd/ND@G catalyst annealed at different temperatures
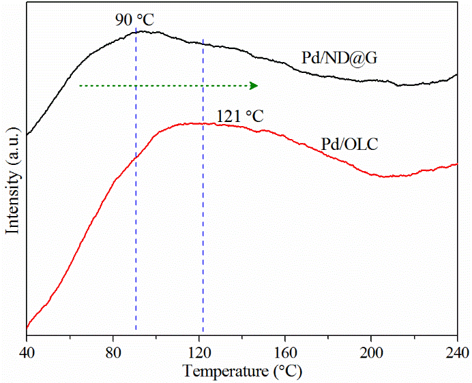
Figure 4. CO TPD spectra for Pd/ND@G and Pd/OLC