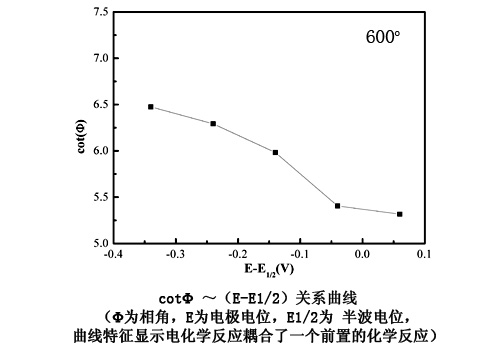
Marine corrosion of turbine blades in planes or ships is very serious because of the synergistic effect of solid NaCl and water vapor at medium and high temperatures. In the last decade, many related researches were carried out in the State Key Laboratory for Corrosion and Protection. Prof. Wang Fuhui has pointed outthat there may be a “dynamic water film” on the surface of the material. An electrochemical corrosion occurs in this water film and accelerates the metal oxidation heavily. Prof. Li and her colleagues studied the electrochemical corrosion behavior of pure Fe and pure Cr in the same environment by using electrochemical impedance spectroscopy (EIS) and published the first paper in the world about this topic in Electrochimica Acta,51(2006)4736-4743. Recently, they make new progress in this study. They found that the whole corrosion process is a CE process (an electrochemical reaction coupled with a preceding chemical reaction), in which Fe first reacts chemically with an NaCl and water vapor to generate HCl(g) and then proceeds via a one-electron electrochemical reduction to form H2. The related results has been published on the Electrochemistry Communications,12 (2010) 191–193.
provided by "State Key Laboratory of Metal Corrosion and Protection"