Photocatalysis is a very promising process for solar-to-chemical energy conversion. The prerequisite for realizing the practical applications of photocatalysis is to develop photocatalysts capable of efficiently utilizing full visible light (λ: 400-700 nm). Most stable photocatalysts, however, suffer from no or low visible light absorbance. Doping is a general method of increasing visible light absorption by narrowing the bandgap. Anatase TiO2 is the most widely investigated photocatalyst in the past four decades. Although doping can increase visible light absorption of TiO2, few success of harvesting the complete spectrum of visible light is achieved.
Shenyang National Laboratory for Materials Science (SYNL), Institute of Metal Research (IMR) has been devoted to solving the challenge of narrowing the bandgap of wide-bandgap semiconductors so as to harvest the complete spectrum of visible light. Their early studies have revealed that the spatial distribution of dopants in photocatalysts plays a vital role in determining the bandgap narrowing. Namely, surface doping results in some localized states in the band gap without narrowing the bandgap, and only bulk doping can cause the bandgap narrowing. Furthermore, it is confirmed that layered structure is favorable for realizing homogeneous doping by providing the facile diffusion channels of dopants. For example, the homogeneous nitrogen doping in layered Cs0.68Ti1.83O4 can realize the redshift of light absorption edge by 100 nm towards visible light region. However, the effective bulk doping in non-layered photocatalysts (i.e. TiO2) remains a challenge.
Recently SYNL developed a new strategy of realizing effective bulk doping by weakening the nearby metal-oxygen (M-O) bonds with interstitial heteroatom for easily substituting lattice O. The resultant anatase TiO2 with a gradient of dopants can absorb the complete spectrum of visible light. Photoelectrochemical water splitting measurements suggest that the action responsive range of anatase TiO2 is extended up to 700 nm.
The challenge of realizing bulk doping originally stems from both the high bonding energy of metal M-O bonds and the charge difference between lattice oxygen and dopant atoms (i.e., O2- versus N3-) in oxide-based photocatalysts. The key to solving this conundrum should have the dual functions of both weakening M-O bonds and compensating for the charge difference. By using crystalline TiB2 as precursor, they first obtained anatase TiO2 microspheres with an interstitial boron gradient with its maximum on surface. Theoretical studies suggest that the interstitial boron can effectively weaken the nearby Ti-O bonds. As a result, the energy for substituting lattice O in the weakened Ti-O bonds is largely decreased compared to that in TiO2 without interstitial boron. Moreover, the stability of nitrogen doped TiO2 is improved due to the existence of boron. Experimentally, it is confirmed that nitridation of anatase TiO2 microspheres with the interstitial boron gradient can result in the effective substitution of N for lattice O in the bulk. The substitutional N has a similar gradient to interstitial boron as a result of the spatial directing effect of the interstitial boron. Meanwhile, the extra electrons contributed by the interstitial boron can compensate the charge different between O2- and N3-.
The resultant anatase TiO2 with a gradient of B/N dopants is red and has a high absorbance in the complete spectrum of visible light (Fig. 1). The photoelectrode fabricated from the red TiO2 has the ability of splitting water up to 700 nm, which indicates the possibility of realizing efficient visible light photocatalytic water splitting with TiO2 based photocatalysts.
The results obtained may shed some light on how to effectively increase high visible light absorbance of wide-bandgap photocatalysts based on the doping strategy. Related results have been published in Adv Funct Mater (2012, 22, 3233-3238), Energy & Environmental Science (2012, DOI:10.1039/C2EE22930G).
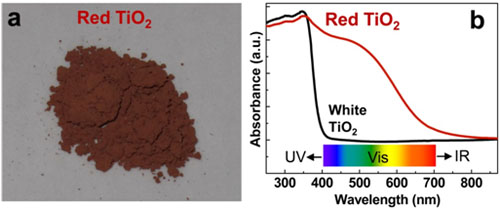
Figure 1. a, photograph of red TiO2; b, UV-visible absorption spectra of white TiO2 and red TiO2. (Image by IMR)
Paper link:
http://onlinelibrary.wiley.com/doi/10.1002/adfm.201200414/abstract
http://pubs.rsc.org/en/Content/ArticleLanding/2012/EE/c2ee22930g