Photocatalysis as a promising process for solar-to-chemical energy conversion provides a possible way of acquiring renewable energy. The prerequisite for realizing the practical applications of photocatalysis is to develop photocatalysts capable of efficiently utilizing visible light. Both modifying known photocatalysts and exploring unknown photocatalysts are significant in developing visible light responsive photocatalyst.
SYNL, IMR has been devoted to developing photocatalysts with a wide visible light response both by narrowing the bandgap of wide-bandgap semiconductors and exploring unknown photocatalysts. Recently they showed that elemental sulfur and boron can act as visible light responsive photocatalysts.
Elemental sulfur has more than 30 allotropes, most of which consist of cyclic molecules with ring sizes of 6−20 sulfur atoms. Among them, S8 is the most stable configuration at standard temperature and pressure (STP) and can crystallize to form the most stable orthorhombic α-sulfur (α-S) at STP (Fig. 1a). α-S crystals with a bandgap of 2.79 eV can absorb visible light up to 475 nm (Fig. 1b) and have suitable band edges for photocatalytic reactions (water splitting and OH radical generation). Further studies showed that α-S crystals have the ability of photoelectrochemical water splitting, generating OH radicals and decomposing Rhodamine B. Decreasing the particle size led to the photocatalytic activity improvement. Furthermore, α-S based photocatalysts have good stability.
Boron has aroused wide research interest owing to its fascinating properties (light weight, high strength, high hardness, high melting point, high chemical resistance, typical semiconductivity, and superconductivity at high pressure). However, the application of boron associated with its semiconducting properties has been rarely explored. β-rhombohedral boron as the most thermodynamically stable form of elemental boron consists of 107 atoms in the unit cell (Fig. 1c). β-rhombohedral boron as p-type semiconductor has a wide light absorption edge up to 800 nm (Fig. 1d). β-rhombohedral boron crystals with submicron size has the ability of stable OH radical generation under visible light. By removing around 2 nm thick surface amorphous layer, the photocatalytic activity can be improved by a factor of 2.2. This is because the localized states or band tails generated by amorphous layer lowers the mobility and redox ability of photoexcited charge carriers.
Related results have been published in J Am Chem Soc 2012, 134, 9070−9073, Angew Chem Int Ed 2013, 52, 6242-6245, ChemPhysChem 2013, 14, 885-892.
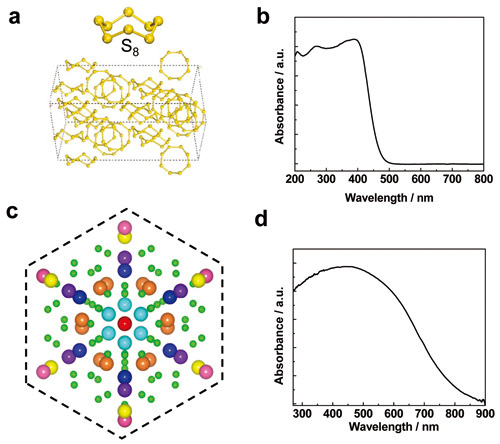
Figure 1 a, the unit cell of α-S crystal; b, UV-visible absorption spectrum of α-S crystals; c. the unit cell of β-rhombohedral crystal; d, UV-visible absorption spectrum of β-rhombohedral crystals.
Contact:
Prof. LIU Gang
Email: gangliu@imr.ac.cn