Sulfur as a cathode material can react with lithium to form Li2S with a two-electron reaction process, leading to a high theoretical capacity of 1675 mAh g−1 and energy density (2600 Wh kg−1) in a lithium-sulfur (Li-S) battery, which is more than 7 times that of commercial lithium-ion batteries (LiCoO2 cathode and graphite anode with a theoretical energy density of 360 Wh kg−1). Sulfur is also inexpensive, abundant and non-toxic. Therefore, Li-S batteries are considered as promising next-generation high-energy batteries.
However, the practical application of Li-S batteries is greatly hampered by three major challenges including (1) the low electrical conductivities of sulfur, the intermediate polysulfide products and the final Li2S product affect the utilisation of the sulfur material and the rate capability of the battery; (2) highly soluble polysulfides in electrolyte, which will shuttle between the anode and cathode and form a deposit of solid Li2S2/Li2S on the cathode and anode (shuttle effect), cause an irreversible loss of sulfur, which leads to low coulombic efficiency, low cyclic capacity and an increase in impedance; (3) a volume change (80%) between sulfur and Li2S during charge/discharge due to the different densities of sulfur and Li2S, induces stress in the electrode and destroys its structural stability, which leads to rapid capacity decay. Thus the sulfur cathode is one of the bottlenecks that need to be addressed before Li-S batteries find practical use. Efforts have been devoted to impregnating sulfur into various porous carbon matrixes, which can not only improve the electrical conductivity but also capture the soluble polysulfides. However, the synthesis of carbon–sulfur composites usually requires elaborate procedures and its complex fabrication processes compromise the utilisation efficiency of sulfur. The addition of carbon materials also reduces the energy density of Li-S batteries and practical battery applications require more than 70 wt% sulfur loading.
Since 2010, a research group at Advanced Carbon Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences made use of carbon materials with high electrical conductivity, good chemical and thermal stability, large surface area, rich surface chemistry to conquer the above challenges. They designed and developed a series of high-performance carbon-sulfur composite cathode materials at nano-scale by confining sulfur in micropores, defects and surface active sites, which effectively improve the electrical conductivity and restrict the polysulfide dissolution.
Most recently, the team designed a unique sandwich structure with pure sulfur between two graphene membranes. One graphene membrane was used as a current collector (GCC) to replace commercial Al foil with sulfur coated on it as active material, and the other graphene membrane was coated on a commercial polymer separator (G-separator). The flexible and conductive graphene layer on the sulfur electrode provides excellent electric conductivity, and the sandwich structure can accommodate the large volumetric expansion of sulfur during lithiation. The presence of the graphene layer on the separator surface acts as a good barrier to mitigate the shuttle effect of dissolved polysulfides. Furthermore, the surface roughness of the GCC can improve the adhesion of sulfur to it and lower the impedance and polarisation of the Li–S battery. These properties provide the Li-S batteries with long cycling life and excellent rate performance. This design avoids any surface modification of sulfur particles and simplifies the fabrication of sulfur cathode. Because the density of a GCC is only one quarter of that of an Al-foil current collector, the use of GCC can further improve the specific energy density when assembled into a battery. Furthermore, for the first time they used a three-dimensional X-ray microtomography to explore the sulphur distribution in the electrode after cycling. It should be noted that the graphene used in the research is produced through an intercalation-exfoliation method from graphite and is commercially available at a low cost. These features give the design and materials strong potential for the industrial production and application of Li–S batteries.
The related work was published in Adv. Mater., 2014, 26, 625–631 (selected as Back cover), ACS Nano, 2013, 7, 5367–5375, Energy Environ. Sci., 2012, 5, 8901–8906, and applied three Chinese patents. The work above were financially supported by the National Science Foundation of China, Ministry of Science and Technology and Chinese Academy of Sciences.
Link to the original paper: 1 2 3
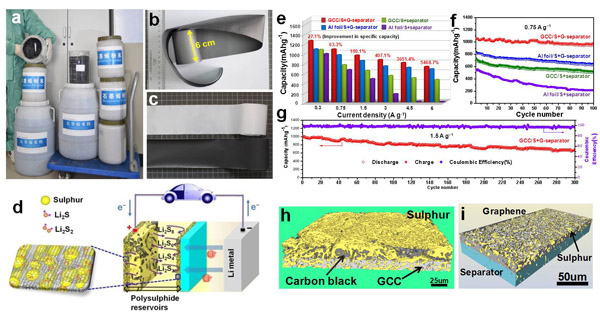
Figure 1 a, Photograph of graphene slurry and powder as industrial products. b, Photographs of an as-prepared large-area GCC strip (width: 6 cm) showing its good flexibility. c, Photograph of a large-area G-separator and a commercial polymer separator. d, Schematic of a Li–S battery with electrode configuration. e, Rate performance of the Li–S batteries with different configurations at different current densities. f, Cycling stability of the Li–S batteries with different configurations at 0.75 A g−1. g, Cycling stability of the Li–S batteries at 1.5 A g−1. h-i, 3D image of the reconstructed sulfur electrode and G-separator.
CONTACT:
LI Feng
Institute of Metal Research, Chinese Academy of Sciences
Email: fli@imr.ac.cn