Fuel cells, as a clean and efficient power source, have attracted significant attention during the last decades. The cathodic oxygen reduction reaction (ORR) is at the heart offuel cell performance, and efficient ORR electrocatalysts areessential for practical applications.The cost of widely used platinum catalysts is prohibitive in fuel cells, so the development of Fe/N/C non-precious metal catalysts with high ORR activity and stabilityhas become a major focus of fuel cell research and become the potential candidates of Pt-based catalysts. The actual structures of active sites in Fe/N/C electrocatalysts remain elusive, because the Fe/N/C electrocatalysts are inherently highly heterogeneousand have complex structure.
Prof. SU Dangsheng and PhD student ZHU Yansong from Catalysis and Materials Devision (Shenyang National Laboratory for Materials Science) show for the first time the N6-coordination of the active Fe-N complexes confined in complex carbon-based electrocatalysts. The information is validated by combining advanced electron microscopy and Mössbauer spectroscopy methods. Taking account of the inherently highly heterogeneous and complex structure of the real Fe-N-containing electrocatalysts, we have excluded the likelihood of other iron-containing phases as ORR active sites, such as Fe3C or FeS.The related research results are published online on AngewandteChemie International Edition(DOI: 10.1002/anie.201405314)in August.
The knowledge of the real active site FeN6 will lead the way totarget-specific synthesis ofhighly active and stable Fe/N/Ccatalysts for ORR. The authors acknowledge the financial support from MOST (2011CBA00504), NSFC of China (21133010, 21203215, 51221264, 21261160487), and ‘Strategic Priority Research Program’ of theChinese Academy of Sciences, Grant No. XDA09030103.

Figure 1 a) STEM image b) HRTEM image of PpPD-Fe-C c) HRTEM image d) HAADF-STEM maps of Fe-N-C (Image by IMR)
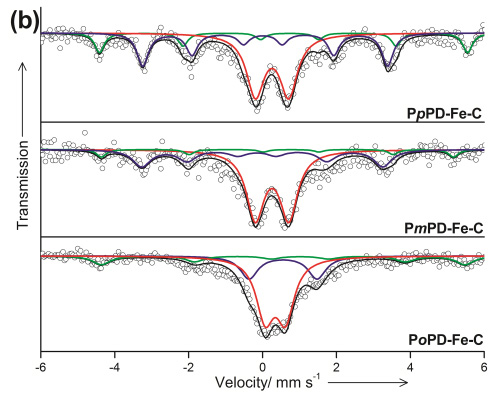
Figure 2 Mçssbauer spectra forFe-N-C (Image by IMR)
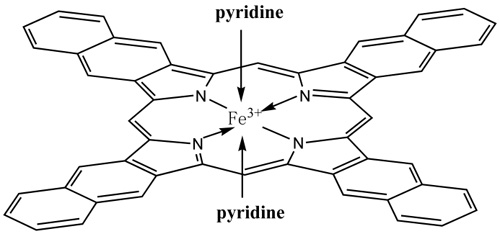
Figure 3 Iron complex structure (Image by IMR)