Ionic liquids (ILs)are liquid organic salts at ambient temperature which are composed of ions. Their polarity, lipophilicity, hydrophilicity and catalytic activity can be readily modified by a suitable choice of cation and anion, making them a series of “designable solvents”. ILs exhibit many properties which make them potentially attractive media for fundamental research, such as low vapor pressure at atmospheric pressure, high thermal and chemical stability and wide liquid range, good solubility for a wide range of both inorganic and organic materials, non-flammability and no ignition point. Although with so many excellent properties, the large-scale applications of ILs are restricted by some disadvantages including high price, low purity and difficulty to separate.
Recently, researchers of Shenyang National Laboratory for Materials Science, the Institute of Metal Research, Chinese Academy of Sciences have developed a new way to synthesize ILs and nano-carbon composites with the interesting interaction force between them. By varying annealing time and temperature, nanometer-scale host-guest structure with different IL contents can be obtained. (Chemsuschem, 2014, 7, 1542–1546) The nanocomposites maintain the properties of both ILs and nano-carbon after the simple physical processes. This new nanocomposite can be used both in carbon catalysis and IL catalysis. The dielectric constants of the ionic liquids can prevent the detached nano-cabon from rebundling, which is favorable for their contact area with the reactants.
In addition, it has been found the condensed IL on carbon surface can be used to fix mono-dispersed catalyst. A new concept of ionic liquid (IL) mono-dispersing catalyst on nanocarbon surface is proposed. The condensed IL phase not only provides a homogenous reaction micro-environment but also lowers the binding energy of the W4f electron of the catalyst leading to an easier reaction with the oxidant. With low dosage of IL and the superiority in catalytic redox reaction, this novel heterogenizat- ion process sheds a light on research on the catalyst-IL interaction and goes a step further to bridge homogeneous-heterogeneouscatalysis (Green Chemistry, 2014, DOI: 10.1039/C4GC01814A).
The strong interaction between ILs and graphitic support allows the entrapping and directing of ILs on nanocarbon and thus the synthesis of heteroatoms-rich carbon materials with non-cross-linkable functional ILs. The structure of obtained surface can be tailored from condensed IL ion pairs to the polymeric, then to the graphitic structures. The properties of the formed nanocarbon surface, such as the hydrophobicity–hydrophility, surface potential, and acid–base character, can be easily tuned by changing the treatment conditions. Heteroatoms are fully exposed on the surface, rendering a high atomic effecting. A few layers and even single layer can be obtained on the surface. Moreover, the template removal process is no needed. All the above advantages bring not only an economical potential application of ILs, but also a fundamental understanding of the nanocarbon and IL interface. The numerous kinds of ILs will undoubtedly give extensive heteroatom candidates for the method. (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201408201)

Figure1 .left: a image of IL/CNT gel and nanocomposites b, c)TEM image of HHT and nanocomposites;right:Schematic representation of the nano-composite. (Image by IMR)
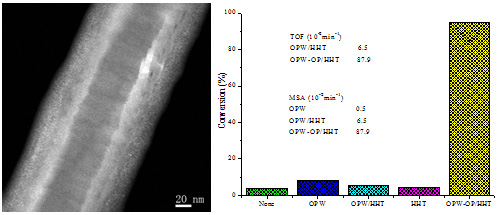
Figure 2. left:HAADF-STEM of OPW-OP/HHT;right:DBT conversion with the same amount of catalysts, TOF and MSA of different catalyst systems (Image by IMR)

Figure 3. The optimized physisorption structure of the cation on graphene. (Image by IMR)