Combustion catalysts have been extensively explored to reduce the emission of hydrocarbons that are capable of triggering photochemical smog and greenhouse effect. Palladium as the most active material is widely applied in exhaust catalytic converter and combustion units, but its high capital cost stimulates the tremendous research on non-noble metal candidates. Extensive research has been performed in searching candidates for noble metal catalysts, but there is still a large demand for practically useful and acceptable catalysts for a variety of important reactions.
The team by Professor ZHANG Zhidong, Professor SU Dangsheng, Associate Professor MA Song (Institute of Metal Research, CAS) and Professor ZHANG Jian (Ningbo Institute of Industrial technology, CAS) fabricated highly defective Co3O4 nanocrystals via a controllable oxidation of carbon-encapsulated cobalt nanoparticles. Strain gradients induced in the nanoconfined carbon shell result in the formation of a large number of active sites featuring a considerable catalytic activity for the combustion of a variety of hydrocarbons (methane, propane and substituted benzenes). For methane combustion, the catalyst displays a unique activity being comparable or even superior to the palladium ones. The present work is published on the website of Nature Communications (DOI: 10.1038/ncomms8181).
On the basis of this concept, the team will explore new technique to synthesize more transition metal oxides for enhancing the reactive efficiency in catalytic combustion reaction of C1~C4 hydrocarbons.
Contact:
Professor ZHANG Jian: jzhang@nimte.ac.cn
Associate Professor MA Song: songma@imr.ac.cn
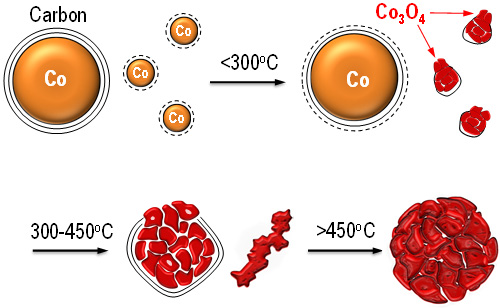
Figure 1. Scheme for the transformation process of the Co3O4 catalyst.(Image by IMR)
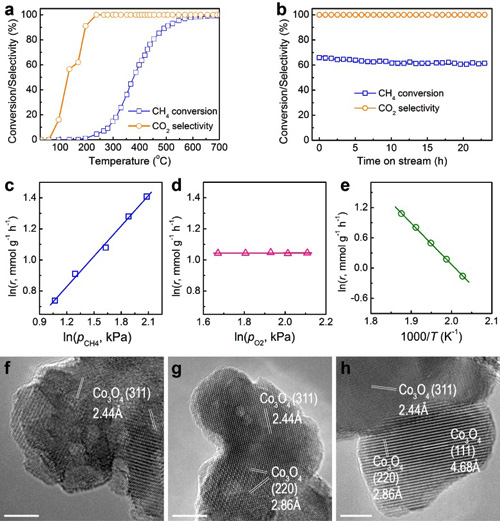
Figure 2. Catalytic performance in CH4 catalytic combustion (a-e) and high-resolution TEM images of the reacted catalyst (f-h). (Image by IMR)