Light alkanes, including C2~C6 paraffins, are abundantly present in natural gas, associated gas, shale gas, and co-products of oil refining, which are relatively inert and underutilized, being often used as low-valued fuels. However, the corresponding light olefins are extensively used as the basic building blocks for plastics, rubbers, resins, macromolecular polymers, agrochemicals, pharmaceuticals, organic chemicals, fine chemicals and petroleum additives, which are facing a booming market and a demanding at 259 million tons in 2016. Therefore, the highly efficient conversion from light paraffins to corresponding olefins would accelerate the use of untraditional gas as a complement feedstock for fossil fuel, which are widely portrayed as the landmark events in the 21st century.
The research team of Prof. SU Dangsheng from Catalysis and Energy Materials Division, Institute of Metal Research, Chinese Academy of Sciences has been dedicated in exploring the fundamental principles and the industrial applications of the catalytic conversion from light paraffins to olefins. In 2008, they found that nanocarbon could be acted as a metal-free catalyst for the oxidative dehydrogenation (ODH) of alkanes, showing higher activity, coking resistance and outstanding stability comparing with conventional noble metal or metal oxide-based catalysts (Science, 2008, 322, 73). Nanocarbons as metal-free catalysts have attracted wide attentions in the conventional thermocatalytic fields. Subsequently, they confirmed, by using model compounds (J. Am. Chem. Soc., 2009, 131, 11296) and chemical titration methods (Angew. Chem. Int. Ed., 2013, 52, 14224), that the ketonic C=O groups are selectively responsible for the dehydrogenation of alkanes. The catalytic cycle was proposed when the nucleophilic oxygen in ketonic groups easily attacks the hydrogen atom in alkanes resulting in the rupture of C-H bonds to yield alkenes, since the reduced hydroquinone groups are oxidized and recovered by oxygen into quinone groups. However, the molecular oxygen are easily dissociated by the edge/defects of carbon surface into electrophilic oxygen species (O-, O22-) to attack the electron-rich C=C bonds in alkenes and thus resulting in the overoxidation and a low alkene selectivity.
Recently, the team of Prof. SU Dangsheng revealed that nonmetal elements of nitrogen, phosphorus and boron could be used as a modulator of the electron density on the carbon surface to regulate the catalytic properties of carbocatalysts in different extent. The electron-rich nitrogen atoms with a similar radius as C atoms can be doped into the framework of carbon nanotubes (CNT) by using CVD methods, and which could provide additional electrons for the graphitic lattice. The graphitic nitrogen (NG) plays a determining role in enhancing their activity by speeding up the activation of oxygen and decreasing the overall activation energy of the reaction, giving a quantitative description as: Ea=117.2-32.7 NG (Chem. Commum., 2013, 49, 8151). Phosphorus atoms have incompletely filled p-orbitals to enable accommodating and controlling surplus electrons from the edge/defect of carbon surface and the oxygen functional groups. With the increase of phosphorus content, the selectivity of alkenes in the ODH of isopentane over phosphorus-modified CNTs presents a volcanic distribution (Fig. 1). In addition, the rising tendencies of the oxidation resistance of phosphorus-modified CNTs are in agreement with the declining trends of substrate consumption, and showing a corresponding tendency (Fig. 2A). With the help of TPD (Fig. 3A, B), ATR-IR (Fig 3C), and kinetic measurements, a conceivable mechanism of the role of phosphorus-modified CNTs in the ODH of isopentane was proposed as shown in Fig 3D: with the increase of phosphorus content, defect sites, where the highly active oxygen species (superoxide O2-, per-oxide O22-) could be formed, and blocked firstly, thus resulting in the reduce of overoxidation and the improvement of alkene selectivity. The alkene selectivity was doubled and outperformed the metal-based catalyst of V-Mg-O. With the continuous increase of phosphorus content, the oxygen functional groups and phosphorus on carbon surface formed the phosphate-ester structures (C-O-PO3), and thus leading to the inhibition of the dehydrogenated active sites and the decrease of alkene selectivity. Finally, excessive phosphorus loading resulted in a full coverage of active sites from CNTs and no paraffin conversion. Boron atoms have incompletely filled p-orbitals similar as phosphorus atoms, but the role of boron is significantly different from that of phosphorus atoms. With the increase of boron content, the improvement of the oxidation resistance of boron-modified CNTs(Fig. 2B), the decline trends of isopentane consumption (Fig 2B) and the increase of alkene selectivity (Fig 1) presented a platform distribution, indicating that the boron modification is more selective than phosphorus towards the blockage of surface defects on CNTs. In addition to CNTs, nanodiamonds have been also modified by boron or phosphorus, which have shown an enhanced dehydrogenation selectivity in the ODH reaction of propane. These studies not only revealed the intrinsic reaction mechanism at atomic level but also shortened the distance between the fundamental researches and the industrial applications.
These works have been published on ChemSusChem (2014, 7, 3476), Catalysis Today (2015, 249, 161), ACS Catalysis (2015, 5, 2436) and Chemical Communications (2015, 51, 9145), which are financially supported by National Natural Science Foundation of China (No. 51221264, 21303226), the National Basic Research Program (973 Program, No. 2011CBA00504), and the Sinopec China No. 21203214.
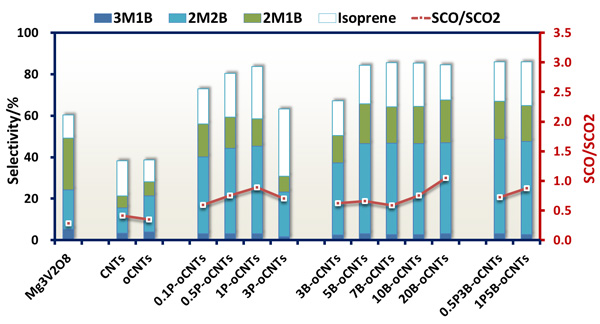
Fig. 1 Product distributions of the ODH reaction of isopentane over different boron- and phosphorus-modified CNTs (Image by IMR)
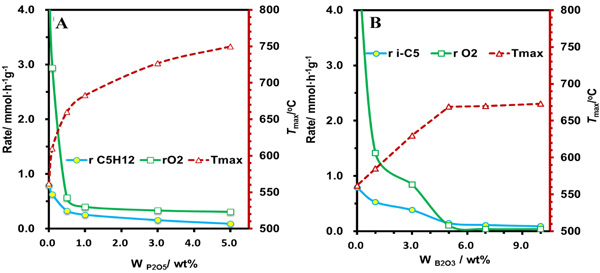
Fig. 2 The temperature of oxidation resistance of phosphorus- (A) and boron-modified (B) CNTsand the reaction rate of substrates in the ODH reaction of isopentane (Image by IMR)
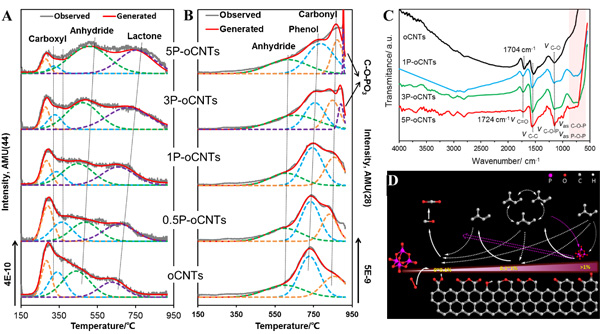
Fig. 3 TPD profiles (A, B), ATR-IR spectra (C) of phosphorus-modified CNTs, and schematic illustrations for the ODH reaction of isopentane (D) (Image by IMR)