Nanocarbon materials have shown stable and efficient catalytic performancein alkane oxidative dehydrogenation (ODH) reactions, and they are considered as one of the most promising alternatives for the conventional noble or toxic metal based catalysts form the viewpoint of sustainable and green chemistry because of their earth abundance and recyclability. With the rapid development of the concept of metal-free catalysis, the research on carbon catalysis has made significant achievements in novel carbon catalyst synthesis andits applications in various important chemical reactions. However, there is only a relatively slow progress in the atomistic understandings on the nature of carbon catalytic process because of the complicated surface structure of nanocarbon, which has seriously restricted the growth of metal-free carbon catalysis.
Recently, the research team ledby Prof. SU Dangsheng and Dr. QI Wei (Catalysis and Materials Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences) has made significant progress in the quantification of the intrinsic activity of nanocarbon catalysts and the molecularly understandings on carbon catalytic process. Firstly, the quantification of the active sites for ODH reaction is successfully realized through the proposed novel in-situ titration process. The titration is based on the selective reaction between the ketonic carbonyl groups on nanocarbon materials and the titrant phenyl hydrazine (PH) molecules (as shown in Fig. 1). With the introduction of titrant PH molecules onto the nanocarbon catalysts under steady state, the apparent activity of the catalysts obviously drops. The linear dependence of the substrate (ethyl benzene) conversion rate as a function of the cumulative consumption of the active sites provides firm chemical evidence on the intrinsic activity of nanocarbon catalysts (as shown in Fig. 2). It is the very first report elaboratesthat the intrinsic catalytic activity of nanocarbon materials could be measured through a single in-situ process. The comparison of the intrinsic activity of nanocarbon catalysts with various morphologies suggests that the microscopic structure of nanocarbon has no obvious effect on their intrinsic activity (as shown in Fig. 3), at least in the scale where the diameter of the nanocarbon materials is above 5 nm. This research shows the importance of exploring the intrinsic activities of nanocarbon catalysts,which is fundamental in the field of carbon catalysis to carryout detailed kinetic studies and to fairly compare catalystreactivity. The corresponding research is published on Angew. Chem. Int. Ed. 2015, 54, 13682-13685.
Prof. SU Dangsheng’s research group pioneered the kinetic and mechanistic studies on non-metallic carbon catalyzed reactions. Their continuous progress on the identification of the catalytic active sites on nanocarbon (Science, 2008, 322, 73; Angew. Chem. Int. Ed., 2013, 52, 14224), the adjustment of the selectivity of carbon catalyzed ODH reactions (Chem. Commun. 2013, 49, 8151) and the basic rules for the rational design of novel nanocarbon catalysts with high efficiency (Chem. Rev. 2013, 113, 5782) have attracted considerable interests in the research community and are accepted and recognized by the researchers working in related field.
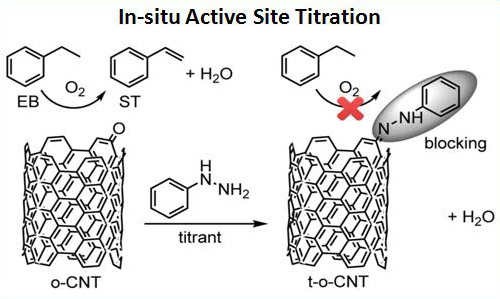
Fig. 1, Schematic drawings of thein-situ titration process for the ODH active sites on nanocarbon catalysts (reprinted from Angew. Chem. Int. Ed. 2015, 54, 13682).
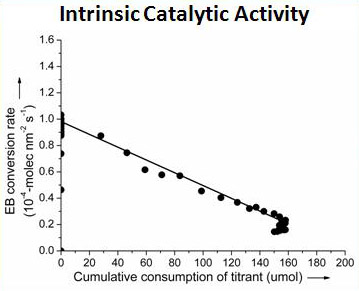
Fig. 2, EB conversion rate as a function of the cumulative consumption of PH titrant (reprinted from Angew. Chem. Int. Ed. 2015, 54, 13682).
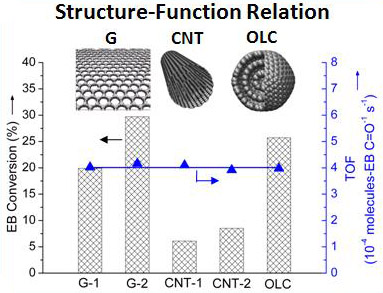
Fig. 3,Comparisons of the ODH activity for various nanocarbon catalysts. EB conversion (bar) and conversion rate normalized by the number of active sites (triangle) (reprinted from Angew. Chem. Int. Ed. 2015, 54, 13682).
Full Text: http://onlinelibrary.wiley.com/doi/10.1002/anie.201505818/full
Key words:heterogeneous catalysis· nanocarbon materials · oxidative dehydrogenation ·active sites· kinetics
Contact: Dr. QI Wei, Catalysis and Materials Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 024-83970027,email:wqi@imr.ac.cn