The eutrophication caused by the excessive existence of nitrogen and phosphorus in natural water bodies is now becoming a severe water pollution problem because it could stimulate blue algae blooming in surface/ground water when proper temperature conditions are present. When blue algae blooms happen, blue algae becomes densely concentrated in water and causes oxygen depletion from its high respiration, resulting in the indiscriminate killing of fish/invertebrates in water and the destruction of the local ecological equilibrium. Furthermore, it could kill livestock and cause various health problems to human beings because it contains a variety of cyanotoxins which could be released when it dies or its cell breaks. In the long run, the prevention of natural water eutrophication by the proper treatment of wastewater to avoid the excessive discharge of nitrogen and phosphorus into natural water bodies is the ultimate solution to blue algae blooms, and the blue algae polluted waters must also be treated to ensure the water safety.
To solve the eutrophication problem and the subsequent blue algae pollution, a series of work has been conducted recently by the research group led by Prof. LI Qi from the Environment Functional Materials Division, Shenyang National Laboratory for Materials Science (SYNL), Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) on the removal of both nitrogen and phosphorus from water, disinfect blue algae, and degrade toxic cyanotoxins. A simple and low-cost solvothermal process is developed to synthesize Ce/Zr binary oxide nanoparticles with a series of Ce/Zr ratios, and their phosphate removal performances are investigated. The modulation of Ce/Zr ratio largely affects the structure, crystal size, and surface properties of these Ce/Zr binary oxide nanoparticles, which subsequently results in their different phosphate adsorption performances. The adsorption kinetics study demonstrates that Ce0.8Zr0.2O2 nanoparticle has the best phosphate adsorption performance among these Ce/Zr binary oxide nanoparticles. The phosphate adsorption capacity of Ce0.8Zr0.2O2 nanoparticles is~112.23 mg/g, which is the highest among various adsorbents reported in literature. The adsorption mechanism of phosphate onto Ce0.8Zr0.2O2 nanoparticles is determined to follow the inner-sphere complexing mechanism and the surface –OH groups play a major role in the phosphate removal.
The photocatalytic treatment of water pollutants is believed to be the most practical approach among various advanced oxidation processes due to its better control of the production of •OH radicals and the potential usage of solar irradiation to provide the energy input. A visible-light-activated photocatalyst, palladium-modified nitrogen-doped titanium oxide (TiO2-xNx/PdO), is developed, which demonstrates a highly efficient photocatalytic oxidation performance on various pollutants in water under visible light illumination. The photoctalytic removal of aqueous NH4+-NH3by TiO2-xNx/PdO nanoparticles is investigated, which demonstrates that a high aqueous NH4+-NH3 removal rate of over 90% could be achieved by TiO2-xNx/PdO nanoparticles under only visible light illumination and the production of toxic photocatalytic oxidation by-products of NO3- and NO2- is minimized. For the first time, it is found that the effective photoctalytic removal of aqueous NH4+-NH3 could be achieved in a weak alkaline solution with constant pH at~ 8.Furthermore, TiO2-xNx/PdO nanoparticles demonstrated a superior photocatalytic disinfection effect on blue algae under visible light illumination. Within hours of visible light illumination, chlorophyll a content in blue algae cells is completely removed by TiO2-xNx/PdO nanoparticles, which could be mainly attributed to its leakage due to the severe damages on the cell wall/membrane of blue algae cells occurs in the photocatalytic disinfection process. Such severe damage also causes the leakage of various ions from blue algae cells, which demonstrates that the photocatalytic disinfection of blue algae by TiO2-xNx/PdO nanoparticles under visible light illumination is a preferred irreversible process. TiO2-xNx/PdO nanoparticles also demonstrate a superior photocatalytic degradation capability on Microcystin LR under visible light illumination, which could solve the cyanotoxin contamination problem in some other blue algae treatment practices.
This series of research work is published by Chemical Engineering Journal, 264 (2015) 437 (Cover Paper), Chemical Engineering Journal, 264 (2015) 728, and Chemical Engineering Journal, 268 (2015) 270. This study is supported by the National Natural Science Foundation of China (Grant No. 51102246), the Knowledge Innovation Program of IMR, CAS (Grant No. Y0N5A111A1), the Basic Science Innovation Program of SYNL (Grant No. Y4N56R1161), the Youth Innovation Promotion Association, CAS (Grant No. Y2N5711171), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China.

Fig. 1. The phosphate adsorption isotherm of Ce0.8Zr0.2O2 nanoparticles.
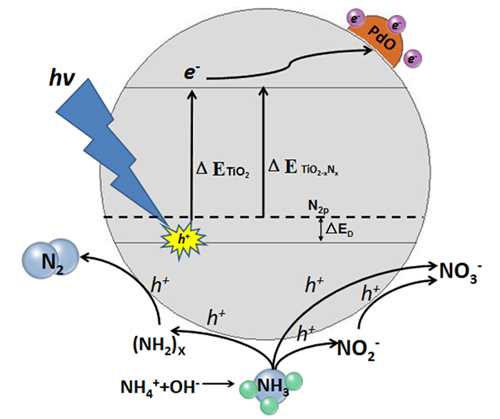
Fig. 2. Mechanism of photocatalytic removal of aqueous NH4+-NH3 by TiO2-xNx/PdO nanoparticles under visible light illumination.
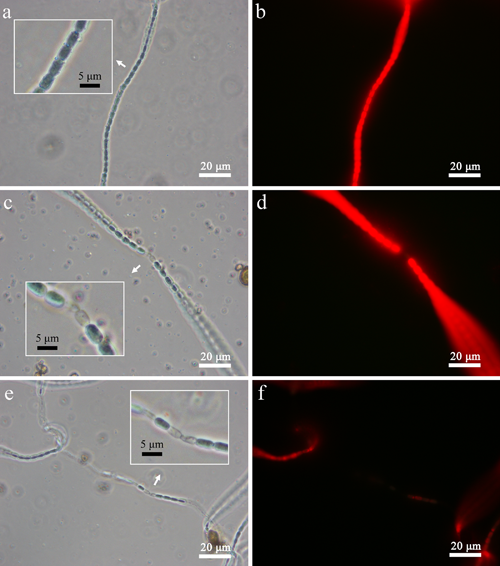
Fig. 3. The real time, in situ phase contrast images and the corresponding fluorescent images of Anabaena sp. PCC 7120 cells treated by TiON/PdO nanopartilces under visible light illumination at different time intervals (a and b: 0 h, c and d: 3 h, and e and f: 6 h).
Contact:
Prof. LI Qi
Email: qili@imr.ac.cn
Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang, Liaoning, 110016, China
Key Words:
eutrophication, blue algae blooms, phosphate adsorption, photocatalytic removal of aqueous NH4+-NH3, photocatalytic disinfection of blue algae, photocatalytic degradation of cyanotoxins, visible light illumination