Since the discovery of photoelectrochemical splitting of water on n-TiO2 electrodes by Fujishima and Honda in 1972, semiconductor-based materials have been investigated extensively as photocatalysts for environmental applications. To make a better usage of the major part of the solar spectrum for photocatalytic reactions and eliminate the cost of UV light source and operation risk with UV illumination, a lot of research efforts are recently made to develop various visible-light-activated photocatalysts with high efficiency. However, most of these visible-light-activated photocatalysts only functions under illumination because their production of reactive oxygen species (ROSs) relies on continuous illumination to generate electron-hole pairs. Thus, they could not treat environmental pollutants continuously only with solar energy, and need secondary light sources to continue their activity at night. The requirement of secondary light sources inevitably increases the cost and energy consumption, while continuous illumination is not appropriate for many potential environmental applications.
To address this problem, Prof. LI Qi from the Environment Functional Materials Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, develops a highly efficient visible-light-activated photocatalyst system which could store some of its photocatalytic activity in “memory” under illumination so that once the photoexcitation is turned off, it could still remain its activity for an extended period of time. Thus, it could make a full use of photon energies from solar illumination or other lighting systems to treat environmental pollutants continuously, which could largely enhance its treatment performance, lower the operation cost and energy consumption, and promote novel applications for photocatalysis on a broad range of environmental applications. Guided by this novel idea, Prof. Li has developed the first generation of photocatalyst with post-illumination photocatalytic “memory” effect, PdO nanoparticle modified, nitrogen-doped titanium dioxide photocatalyst (TiON/PdO). In this TiON/PdO photocatalytic material system, photogenerates electrons trapped on PdO nanoparticles could be released and react with oxygen/water to produce •O2– and •OH radicals when the visible light illumination is switched off, which provides TiON/PdO with the continuous capability to remove various pollutants in the dark (see Advanced Materials, 2008, 20, 3717 and Journal of Materials Chemistry, 2010, 20, 1068).
Based on their previous work, the research group led by Prof. LI makes important progresses recently on the photocatalytic “memory” effect study by investigating its working mechanism and potential radicals effective for it. Their study finds out that the photocatalytic “memory” effect is not limited to photocatalysts with noble metal modifications. It may be possessed by any photocatalyst system, and photo-excited electrons could transfer from the light absorber component to the decoration component through proper matching of their energy band structure while the decoration component could trap and release them in this system. Thus, highly efficient photocatalysts with post-illumination photocatalytic “memory” effect could be developed with enhanced performance and lower cost. For example, they develop the secondary generation of photocatalyst with post-illumination photocatalytic “memory” effect, a composite photocatalyst of Cu2O nano-spheres decorated with TiO2 nano-islands, which is synthesized by a facile hydrolyzation reaction of Cu2O nano-spheres followed by a solvent-thermal process. In this photocatalyst system, p-type Cu2O nano-spheres serves as the main component for light absorption so it demonstrates excellent absorption capability in the visible light region, while n-type TiO2 nano-islands forms p-n heterojunctions of good contact with Cu2O nano-spheres, beneficial to the photogenerated electron transfer between them. The close match of their band structures and the inner electrostatic field from the p-n heterojunction favors the transfer of photogenerated electrons from Cu2O to TiO2, which effectively separates the electron-hole pairs and subsequently enhanced their photocatalytic performance under visible light illumination. These transferred electrons could be trapped and stored on TiO2 nano-islands by reducing Ti4+ to Ti3+, and then be released in the dark to produce •O2– and •OH radicals for its demonstrated photocatalytic “memory” effect (see ACS Applied Materials & Interfaces, 2014, 6, 5629).
In the further study on potential radicals effective for the post-illumination photocatalytic “memory” effect, they find that it is not necessary for trapped electrons to have a high energy to ensure their reaction with oxygen through the one-electron reduction to produce •O2– radicals for the post-illumination photocatalytic “memory” effect. A decoration component with the conduction band potential less positive than the two-electron reduction potential of O2 could be effective to generate activity from the production of H2O2 in the dark, which could not only largely expand the selection of potential decoration components but also increase the conduction band potential difference to enhance the driving force for the photogenerated electrons to be injected from the light absorber component’s conduction band to that of the decoration component for their better transfer, trapping and subsequent release. Thus, the selection of potential decoration components to construct photocatalyst systems with the post-illumination photocatalytic “memory” effect could be largely expanded to more semiconductors, such as SnO2, WO3, CuWO4, BiWO6, CeO2, etc, if their conduction band potentials are less positive than the two-electron reduction potential of O2. Guided by this finding, they developes the third generation of photocatalyst with post-illumination photocatalytic “memory” effect, a composite photocatalyst composes of Cu2O nanocubes decorated with SnO2 nanoparticles. In this Cu2O/SnO2 photocatalyst system, Cu2O serves as the main visible light absorber to produce electron-hole pairs, while SnO2 nanoparticle decoration forms p-n heterojunction of good contact with Cu2O nanocubes. The combined effect from both their large conduction band potential difference and the inner electric field provides a strong driving force for photogenerated electrons to transfer from the conduction band of Cu2O to that of SnO2 and be trapped there under visible light illumination. Thus, a largely enhanced photocatalytic performance is observed on this Cu2O/SnO2 photocatalyst, compared with pure Cu2O nanocubes. When the visible light illumination is turned off, trapped electrons could be released from SnO2 and react with O2 to produce H2O2 in the dark for more than 24 h, and the Cu2O/SnO2 sample demonstrates a strong and extended post-illumination photocatalytic “memory” effect in the dark (see Scientific Reports, 2016, 6, 20878).
The study on the post-illumination photocatalytic “memory” effect demonstrates that a lot of photocatalyst systems could possess this interesting effect, and their working mechanisms and effective radicals may vary. By proper material design, this interesting effect could be modulated and optimized. This study is supported by the National Natural Science Foundation of China (Grant No. 51102246), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, P. R. China, the Youth Innovation Promotion Association, Chinese Academy of Sciences (Grant No.Y2N5711171), the Knowledge Innovation Program of Institute of Metal Research, Chinese Academy of Sciences (Grant No. Y0N5A111A1), and the Basic Science Innovation Program of Shenyang National Laboratory for Materials Science (Grant No. Y4N56R1161).
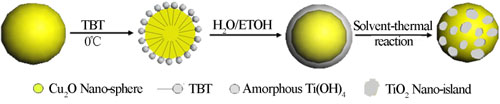
Fig. 1. Schematic illustration of the synthesis process of Cu2O nano-spheres decorated with TiO2 nano-islands.
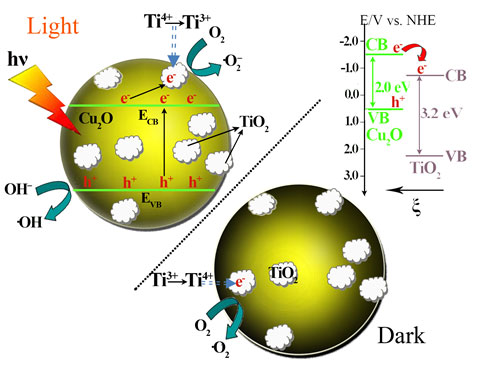
Fig. 2. The proposed energy band structure of the Cu2O/TiO2 p-n heterojunction, the photocatalytic activity enhancement mechanism under visible light illumination, and the post-illumination catalytic “memory” mechanism in the dark.
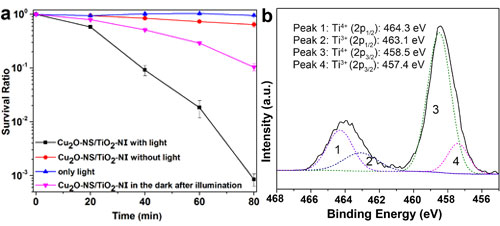
Fig. 3. (a) The survival ratio of E. coli with the treatment by Cu2O-NS/TiO2-NI under visible light illumination, compared with that without photocatalyst under visible light illumination, with photocatalyst in the dark, and with photocatalyst in the dark after illuminated for 3 h first. (b) The high resolution XPS scans over Ti 2p peaks under visible light illumination.
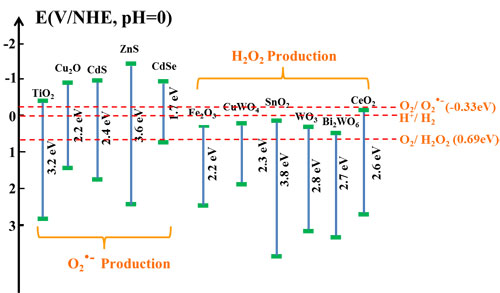
Fig. 4. Schematic illustration of the energy band structure of some common semiconductor photocatalysts.
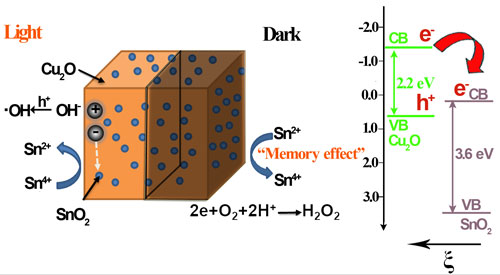
Fig. 5. The proposed energy band structure of the Cu2O/SnO2 p-n heterojunction, the photocatalytic activity enhancement mechanism under visible light illumination, and the post-illumination photocatalytic “memory” mechanism in the dark.
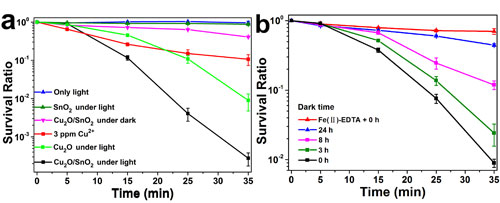
Fig. 6. (a) The survival ratio of S. aureus cells with the treatment by the Cu2O/SnO2 sample under visible light illumination, compared with that without photocatalyst under visible light illumination, that with photocatalyst in the dark, and that by Cu2+ ion with 3 ppm concentration. (b) The S. aureus cell survival ratios in the dark treated by pre-illuminated Cu2O/SnO2 samples after being stored in the dark for various times, compared with that with a H2O2 scavenger, EDTA-Fe(II) (0.1 M), in the S. aureus cell suspension treated by the pre-illuminated Cu2O/SnO2 sample in the dark with the dark storage time of 0 h.

Fig. 7. The H2O2 concentrations in the test solution by the Cu2O/SnO2 sample (black), the as-synthesized Cu2O nanocubes (red), and SnO2 nanoparticles (blue), respectively, in the dark for up to 24 h after being illuminated under visible light for 3 h.
Contact:
Prof. LI Qi
Email: qili@imr.ac.cn
Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang, Liaoning, 110016, China
Keywords: Photocatalytic “Memory” Effect; Visible Light Photocatalysis; Mechanism; Radical; Electron Trapping and Release.