Lithium ion battery is one of the most popular energy storage devices so far, which is mainly composed of cathode, anode, separator, and electrolyte. Among the components, cathode is the key to the battery’s electrochemical performance like power density and cycle life. Much attention has been directed to the representative cathode material, olivine-structured LiFePO4 due to high energy density, excellent thermal stability, attractive cost, and environmental benign over the past two decades. In order to achieve high-rate electrochemical performance, researchers have long been committed to shorten the diffusion distance of lithium ion, that is, to reduce the crystal size along the [010] direction. A recent thought-provoking research pointed out that the electrode is composed of ensembles of particles, and the electrode’s electrochemical performance is ultimately determined by the fraction of actively intercalating particles. Therefore, how to obtain a high number of active particles is a core problem in the research of LiFePO4 as a cathode material.
To solve this problem, the research group led by Prof. WANG Xiaohui from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) for the first time synthesize 12 nm-thick [100]-oriented LiFePO4 nanoflakes by a simple one-pot solvothermal method though creating an acidic synthesis environment as waterless as possible, on the basis of their early studies (J. Phys. Chem. C 114: 16806 (2010); Phys. Chem. Chem. Phys. 14: 2669 (2012); CrystEngCommun 16: 10112 (2014)). As identified by voltage hysteresis experiments, the electrode composed of the [100]-oriented LiFePO4 nanoflakes It is intriguing that the ultrathin [100]-oriented LiFePO4 shows excellent rate performance and cycling stability. It delivers a discharge capacity of 122 mAh g−1 (72% of theoretical capacity) even at a current rate of 20 C (60 min/20 = 3 min) and retains a capacity of 90% after 1000 cycles at 10 C. This work offers the new idea that decreasing the Li+ diffusion direction along the b axis is not the only way to design high-performance LiFePO4 cathode materials; rather, it can also be achieved by reducing the distance along the a axis through increasing the active population. The results are published in Nano Letters (16: 795 exhibits the smallest voltage gap by far. Besides, potentiostatic intermittent titration technique experiments indicate that both the activation rate and conversion rate in the [100]-oriented LiFePO4 nanoflake electrode are all significantly large. Such experimental results demonstrate a high percentage of active population for the [100]-oriented nanoflake electrode.-799 (2016)).
This work is supported by the Youth Innovation Promotion Association, CAS under Grant No. 2011152 and Shenyang National Laboratory for Materials Science, IMR, CAS.
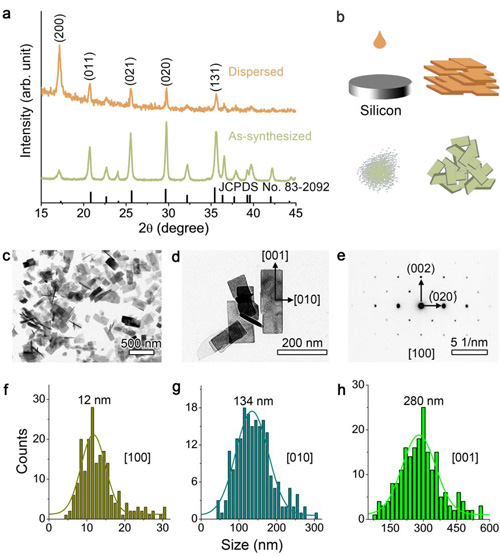
Figure 1. (a) XRD patterns of the as-synthesized particles and those first dispersed in ethanol and then slowly dried on an amorphous silicon substrate. (b) Schematic illustration of the as-synthesized LFP nanoflakes and the dispersed ones. (c, d) TEM images of LFP nanoflakes. (e) SAED pattern corresponding to (d). Statistics of the sizes along the axes (f) a, (g) b, and (h) c using Gaussian fitting.
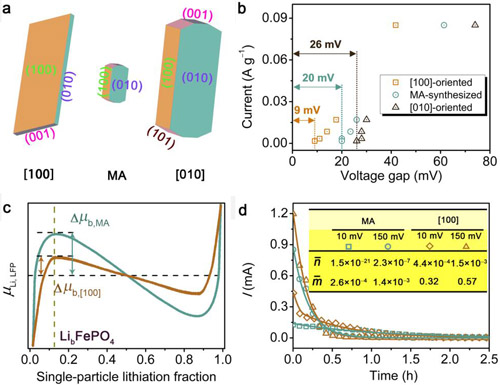
Figure 2. (a) Diagrammatic drawing of ultrathin [100]-oriented, microwave-assisted (MA) -synthesized and [010]-oriented LFP. (b) Voltage gap resulting from hysteresis at different charge/discharge currents in the range from C/2 to C/100 for [100]-oriented and MA-synthesized and [010]-oriented LFP/C electrodes. (c) Chemical potential of Li as a function of the particle’s lithiation fraction. (d) Fitting results of the PITT experimental data of ultrathin [100]-oriented and MA-synthesized LFP.
Contact:
Prof. WANG Xiaohui
Email: wang@imr.ac.cn
Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang 110016, Liaoning, China
Key Words:
Li-ion Battery, Cathode Materials, LiFePO4, Solvothermal, Crystal Orientation
Full Text:
http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.5b04855