Functionalized anilines are important industrial intermediates for a variety of pharmaceuticals, polymers, herbicides and dyes. Nowadays, they are mainly obtained by using a vast of reducing agents, such as sodium hydrosulfite, hydrazine hydrate or some unrecoverable non-noble metal ions, which always lead to serious environmental issues. It is thus of great necessity to explore one environmental-friendly method to efficiently produce anilines. Catalytic hydrogenation using molecular hydrogen (H2) is one of the best choices for the production of functionalized anilines owning to the clean and efficient production process. However, it is difficult to achieve outstanding selectivity for chemoselective hydrogenation of nitroarenes with other reducible groups on the benzene ring.

Figure 1. The application of IL-TEM method in the liquid phase reaction for accurate investigation of structure-evolution of catalysts (left). Highly dispersed Pt NPs supported on the surface modified CNTs and its structure-activity relationship in the catalytic chemoselective hydrogenation of nitroarenes (right). (Imgae by IMR)
To solve this problem, the research group led by Prof. ZHANG Bingsen and Prof. SU Dangsheng from Shenyang National Laboratory for Materials Science (SYNL), the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), made the progress on catalytic chemoselective hydrogenation of nitroarenes through tuning the interaction between surface modified carbon nanotubes (CNTs) and platinum nanoparticles (Pt NPs). The results indicates that the strong metal-support interaction along with the electron-donor capacity of nitrogen-sites on CNTs with a high amount of nitrogen doping (H-NCNTs) are capable of stabilizing Pt NPs and achieving related catalytic recyclability as well as approximately 100% selectivity. The catalyst also delivers exclusively hydrogenation towards nitro groups for a wide scope of substituent nitroarenes into their corresponding anilines. The good stability under reaction conditions is studied by identical location transmission electron microscopy (IL-TEM) method, which is applied to study and compare the catalysts at identical locations at any reaction time in liquid reaction. The result intuitively proves that the introduction of the nitrogen species on the surface of CNTs played an important role on stabilizing Pt NPs. The case suggests a new route for rational designing high-performance catalysts through tuning the interaction between metal NPs and the surface of nanocarbon based supports, and thus enhancing the catalytic hydrogenation performance.
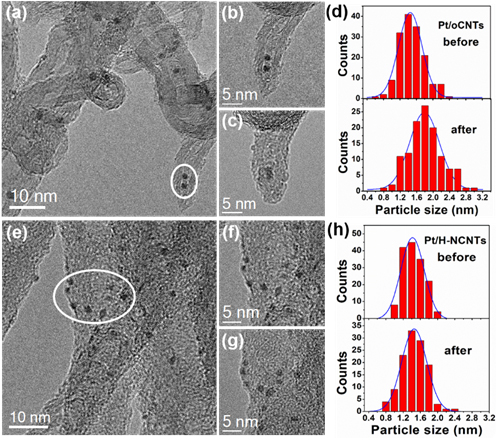
Figure 2. TEM images of Pt/oCNTs (a) and Pt/H-NCNTs (e) catalysts, the enlargement of selected area (outlined in white) before (b, f) and after the reaction for 30 min (c, g), and the corresponding PSD histograms (d, h) by quantifying a series of TEM images at identical locations. (Imgae by IMR)
In recent years, the group has done a series of work on the nanocarbon based materials as supports for nano-scale metal catalysts (Chem Commun 2014, 50, 3856; ChemCatChem 2014, 6, 2607; Chem Commun 2016, 52, 3927). Representative reviews concerning the latest research results as well as the development of this field that provide important references for the rational design of metal/carbon catalyst were also published in the typical journals (Chem Rev, 2015, 115, 2818; ChemCatChem 2015, 7, 3639). The work is supported by the National Natural Science Foundation of China (No.91545119, 21203215, 21133010, 51221264, and 21261160487), the Youth Innovation Promotion Association CAS (2015152), the Joint Foundation of Liaoning Province National Science Foundation and Shenyang National Laboratory for Materials Science (2015021011).
Contact:
Prof. ZHANG Bingsen
Email: bszhang@imr.ac.cn
Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang 110016, Liaoning, China
Full Text(The work was published in ACS Catalysis 【IF=9.3】)