Biomass is organic matter derived from all the living organisms. It most often refers to cellulose, hemicellulose and lignin, which are the main components of plants. 170 billion tons of biomass can be produced every year globally by photosynthesis. The energy contained in these biomass equal to 535.5 billion barrels of crude oils, obviously higher than the world crude oil consumption in 2015 (ca. 35 billion barrels). However, only 3-4% limited biomass is applied, thus the development and application of biomass are very promising.
So far, the research on the conversion of biomass is focused on its conversion to fuels and chemicals. Hydrothermal carbonization, which is generally conducted around 200℃ and has obvious advantages such as mild operation conditions and energy efficient, could convert biomass to functionalized carbon materials. It was reported that the hydrothermal carbon could be efficiently applied in electrochemistry and adsorption of heavy metal ions. However, the application of hydrothermal carbon in catalysis is limited because of its low surface area.
Recently, the oxygen functional groups enriched carbon materials with relatively larger surface area were prepared via hydrothermal carbonization by Dr. WEN Guodong, Prof. TIAN Zhijian and Prof. SU Dangsheng from Institute of Metal Research and Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
The morphology and size could be tuned through changing the initial biomass concentration, adding surfactant, changing the carbonization temperature or adding small molecules with functional groups. In reduction of nitrobenzene reaction, the hydroxyl and carbonyl enriched hydrothermal carbon exhibit higher activity than that of several known carbon materials such as carbon nanotubes, nanodiamond and graphite.
It is found that higher activity could be obtained on sphere morphology with smaller size, on which more active sites could be exposed. In Beckmann rearrangement reaction, the weak Brønsted acid carboxyl enriched hydrothermal carbon gives even higher product selectivity than that of conventionally used solid acid (e.g. zeolite HY and HZSM-5), on which the producing of side products was enhanced on its strong acid sites and weak Lewis acid sites. Although the intrinsic acidity of carbon materials is low, these results indicate that the carbon materials have obvious advantages compared with conventionally used solid acid in some weak Brønsted acid-catalyzed reactions.
This work has been recently published online in Angew. Chem. Int. Ed..
This work is supported by National Natural Science Foundation of China, Doctoral Starting up Foundation of Liaoning Province (China) and CAS Strategic Priority Research Program.
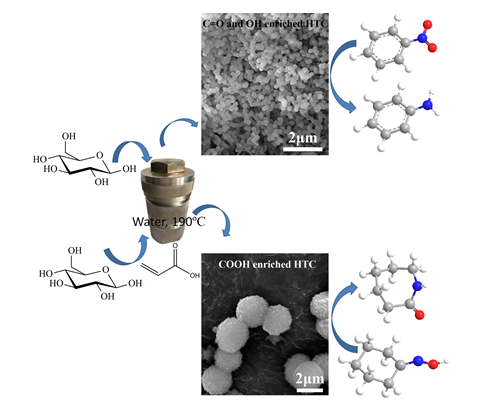
Figure. Oxygenated groups enriched carbocatalysts with different size and morphology were synthesized via hydrothermal carbonization from glucose. The hydroxyl and carbonyl enriched hydrothermal carbon (HTC) could be used as catalysts in reduction of nitrobenzene, higher conversion could be obtained on smaller sphere size. The carboxyl enriched HTC could be efficiently used in weak Br?nsted acid-catalyzed Beckmann rearrangement reaction.(Image by IMR)
Contact:
Dr. WEN Guodong
Institute of Metal Research, Chinese Academy of Sciences
Email: wengd@imr.ac.cn