Since the discovery of photoelectrochemical splitting of water on n-TiO2 electrodes by Fujishima and Honda in 1972, semiconductor-based materials have been investigated extensively as photocatalysts for solar energy conversion and environmental applications. When TiO2 is excited, both reduction and oxidation reactions could happen when photogenerated electrons and holes migrate to its surface and react with substances absorbed on/near its surface. It had demonstrated that photocatalysis could be a promising technology for the removal of aqueous oxoanions from drinking water. However, previous studies on the photocatalytic bromate reduction were usually conducted under UV light illumination, or had limited photocatalytic reduction efficiency. To enhance the photocatalytic reduction efficiency, sacrificial agents are usually needed to deplete the photogenerated holes [12,15,16]. However, it could increase the complexity and cost of the operation, and may not be appropriate for drinking water treatment due to the addition of substances with potential hazard.
For the enhancement of the photocatalytic efficiency, noble/transition metal modification is widely used as the electron trapping center to enhance the photogenerated electron-hole pair separation due to their relatively high work functions. However, this material design strategy could not deplete photogenerated holes with strong oxidation capability, and the addition of sacrificial agents is still needed for the efficient photocatalytic reduction. It would be most desirable to design a photocatalyst system for photocatalytic reduction in which the charge carrier recombination could be minimized by modifications with hole trapping and consumption capability. Thus, the addition of sacrificial agents could be removed to solve problems associated with it.
To address this problem, Prof. LI Qi of the Environment Functional Materials Division, Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, proposed to develop a highly efficient visible-light-activated photocatalyst for photocatalytic reduction in water purification, by which an efficient photocatalytic bromate reduction under visible light illumination was achieved without the addition of sacrificial agents in the reaction solution. Through theoretical analysis and material screening, Bi quantum dots were deposited onto rutile TiO2 nanoparticles by a one-pot, solvent-thermal process to create the Bi/TiO2 (rutile) heterojunction photocatalyst. As a semimetal element (a weak overlap exists between its valence and conduction bands), bismuth may provide the hole trapping and consumption capability. Unlike most metals, bulk Bi has a relatively low work function of ~ 4.22 eV, close to that of TiO2 at ~ 4.20 eV. With its size decrease into the nano range, the quantum confinement could induce a transition from metal to semiconductor on Bi with the moving up of its conduction subbands and moving down of its valence subbands. So photogenerated electrons could not transfer from TiO2 to Bi quantum dots anymore, while photogenerated holes could transfer from TiO2 to Bi quantum dots and be consumed by oxidizing Bi0 to Bi3+. Thus, it could enhance the lifetime of photogenerated electrons for an efficient reduction process, while no sacrificial agents are needed to deplete holes. This study demonstrated a novel strategy for the design of photocatalysts with strong photocatalytic reduction capabilities for a broad range of technical applications (see Applied Catalysis B: Environmental, 2017, 218, 111-118).
This study was supported by the National Natural Science Foundation of China (Grant No. 51672283 and 51602316), the Basic Science Innovation Program of Shenyang National Laboratory for Materials Science (Grant No. Y4N56R1161 and Y5N56F2161), and the “Geping Green Action”-123 Project on Environment Research and Education of Liaoning Province (Grant No. CEPF2014-123-1-4).
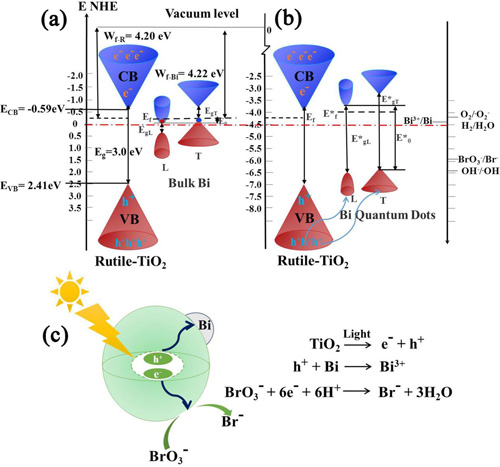
Fig. 1. Proposed energy band structures of (a) Bulk Bi/TiO2 (rutile) and (b) the Bi(1.0)/TiO2 (rutile) photocatalysts. (c) The photocatalytic activity enhancement mechanism under visible light illumination. (Note: Ef refers to Fermi energy, EgL refers to gap energy at L-point of the Brillouin zone, EgT refers to energy gap at T-point of the Brillouin zone, and E0 refers to energy difference between the bottom of the conduction band at the L-point of the Brillouin zone and the top of the valence band at the T-point of the Brillouin zone.)
Corresponding author: Prof. Qi Li
E-mail address: qili@imr.ac.cn
Phone: +86-24-83978028, Fax: +86-24-23971215.
Postal address: 72 Wenhua Road, Shenyang, Liaoning Province, 110016, P. R. China.