Nanocarbon materials have shown great potential in replacing or upgrading conventional noble metal or transition metal oxide catalysts, and are considered as the new generation of green catalysts to meet the urgent and strict demands on environmental protection and sustainable development from the view point of modern chemical industry. However, in-depth mechanistic research in carbon catalysis has grown slowly compared with the unparalleled development of novel nanocarbon catalytic materials and their applications in new reaction systems because of the structural complexity of carbon materials.
Recently, Prof. QI Wei and SU Dangsheng’s group from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) has published a review paper in Accounts of Chemical Research to systemically summarize their research progress in the past 5 years, which built a complete research strategy for the kinetic and mechanistic studies on carbon catalytic process.
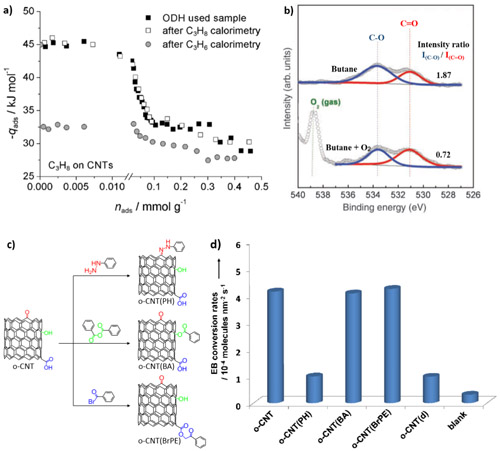
Fig. 1, Research strategy for kinetic and mechanistic studies on carbon catalytic process. (Image by IMR)
The nature of the active sites for carbon catalyzed alkane oxidative dehydrogenation (ODH) reactions has been revealed through microcalorimetric analysis, ambient pressure X-ray photoelectron spectroscopy (XPS) measurement, and in situ chemical titration strategies (Fig. 1). The detailed kinetic analysis and in situ catalyst structure characterization (Fig.2) suggests that carbon catalyzed ODH reactions involve the redox cycles of the ketonic carbonyl-hydroxyl pairs, and the key physicochemical parameters (activation energy, reaction order, and rate/equilibrium constants, etc.) of the carbon catalytic systems are proposed and compared with conventional catalysts, guiding the direction for the rational design of novel nanocarbon materials with high redox catalytic activity.
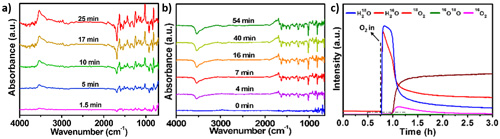
Fig. 2, In situ IR measurements on model carbon catalysts. (Image by IMR)
Actually, Prof. QI Wei's group has been working in the field of carbon catalysis for over 6 years, and this is the 6th straight serial paper for identification and quantification of active sites, intrinsic catalytic activity, kinetics and structure-function relations in carbon catalysis (6 papers with the same main title solving different problems for kinetic studies and IF for these papers all above 10). The research strategy and methods proposed for carbon catalysts may also shed light on other complicated catalytic systems or fields concerning the applications of nonmetallic materials, such as energy storage and environment protection etc.
This work entitled ”Oxidative Dehydrogenation on Nanocarbon: Insights into the Reaction Mechanism and Kinetics via in Situ Experimental Methods” has been published in Acc. Chem. Res. 2018, 51, 640-648. The authors acknowledge the financial support from the NSFC of China (21761132010, 91645114, and 21573256) and the Youth Innovation Promotion Association, CAS.