Associate Prof. LIU Hongyang and his team from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) recently reported an atomically dispersed palladium (Pd) catalyst supported onto a novel nanodiamond-graphene (ND@G) for acetylene hydrogenation. Journal of the American Chemical Society has published this finding online (DOI: 10.1021/jacs.8b07476) and recommended it as cover article.
Selective hydrogenation of alkynes to alkenes, without further reduction to alkanes, is an important reaction for the production of alkenes polymerization, aiming at removing the small number of alkynes in the alkenes monomer. At present, supported Pd catalysts are most widely used for this reaction, but a sharp decrease in the selectivity to alkenes usually occurs over these catalysts when conversion of alkynes is high. Thus, it is vital to design a hydrogenation catalyst with a high activity, high selectivity and good stability.
Associate Prof. LIU and his colleagues are dedicated to develop new nano-carbon material supported metal catalysts. They firstly utilized an atomically dispersed Pd catalyst that consists only of isolated single Pd atoms anchored onto a defective ND@G nanocarbon support (Pd1/ND@G) in selective hydrogenation reaction of acetylene. (Fig. 1). They use aberration-corrected transmission electron microscopy (Fig. 2) and X-ray adsorption fine structure (XAFS) spectra (Fig. 3) confirmed the dispersion morphology, electronic structures and coorcination environments of Pd atoms anchored on ND@G. Also, they built the catalyst structures and studied the reaction mechanism by quuantum chemistry simulation at the DFT level (Fig. 4). The unique structure of the catalyst (atomically dispersed Pd through Pd-C bond anchoring) ensure the facile desorption of ethylene against further hydrogenation to undesired ethane, which is the key for the outstanding selectivity of the catalyst. This work paves the way for rational design of highly selective catalysts for hydrogenation.
This work was supported by NSFC, the Ministry of Science and Technology, the Youth Innovation Promotion Association, CAS, Institute of Metal Research, SINOPEC and Shanghai Synchrotron Radiation Facility (SSRF).
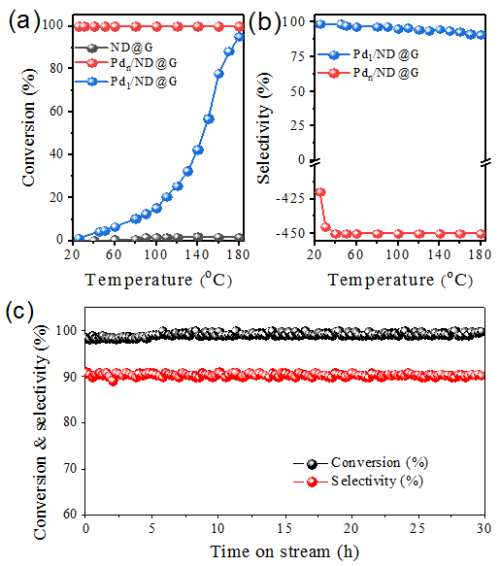
Figure 1. Conversion (a) and selectivity (b) as a function of temperature for acetylene hydrogenation over Pdn/ND@G and Pd1/ND@G catalysts; and durability test on Pd1/ND@G at 180 oC for 30 h (c). (reaction condition: 1% C2H2, 10% H2, 20% C2H4 gas mix balanced with He; GHSV = 60000 h-1.) (Image by IMR)
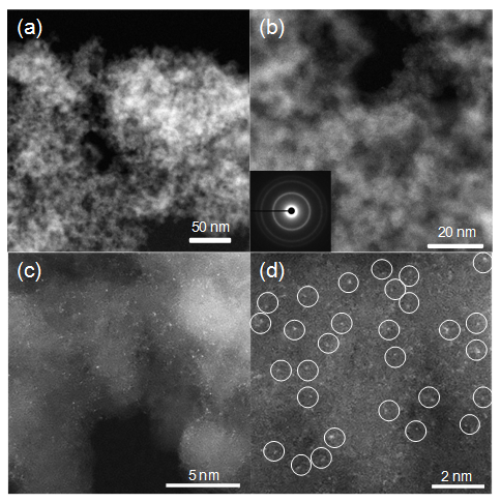
Figure 2. HAADF-STEM images of Pd1/ND@G at low (a, b) and high (c, d) magnifications (Image by IMR)
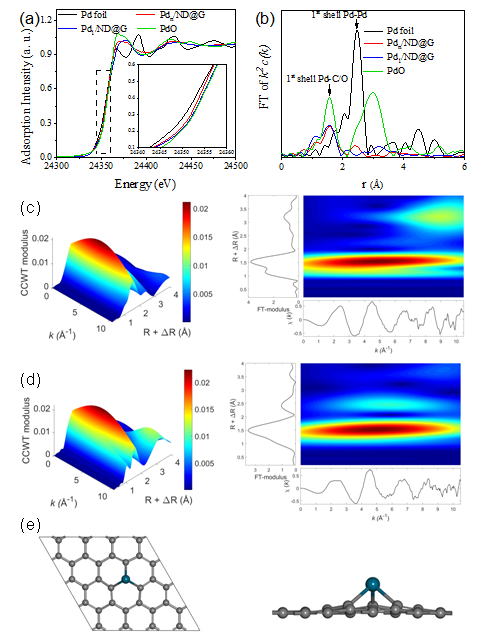
Figure 3. Pd K-edge XANES profiles (a) and EXAFS spectra (b) for Pd1/ND@G and Pdn/ND@G (inset: expansion of the highlighted region); WT analysis of Pd1/ND@G (c) and Pdn/ND@G (d); and the optimized structure of Pd atom embedded into graphene from top (left) and side (right) views (e). Color code: Pd (blue) and C (Dark). (Image by IMR)
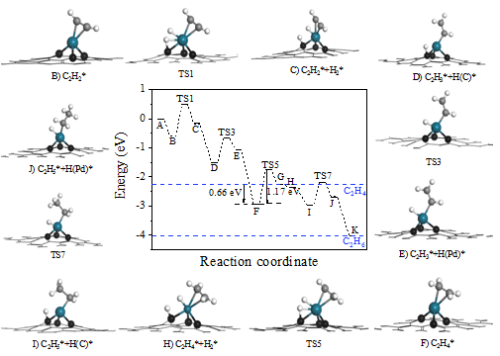
Figure 4. Energy profile of acetylene hydrogenation on the Pd1/ND@G catalyst. Color code: Pd (blue), C in graphene (black), C in reactant/intermediates/product (grey), and H (white). (Image by IMR)

The cover page (Image by JACS)