Alkenes are important industrial monomers which are widely used in the manufacture of polymers, copolymers and reinforced plastics. Many of them are produced by dehydrogenation of the corresponding alkanes. For example, styrene is mainly produced by the direct dehydrogenation of ethylbenzene over multi-promoted iron oxide catalysts under high temperature and excess overheated steam presently. This traditional production method requires a large amount of energy and water resources, which is not conducive to the development of the green economy. Therefore, exploring new catalytic materials with high reactivity and reduced energy consumption has always been the focus of research in the field of industrial dehydrogenation.
MXenes, a newly discovered category of 2D materials named according to their graphene-like morphology, were obtained by selectively etching off the “A” elements from their counterparts, the MAX phases. They often possess versatile chemical composition, tunable layer thickness, and facile functionalization, have been attracting extensive attention in the field of supercapacitors, lithium/sodium ion batteries, electromagnetic shielding materials and so on.
Recently, LIU Hongyang’s group in cooperation with LI Bo and WANG Xiaohui from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) first employed the Ti3C2Tx MXene in the catalytic dehydrogenation of ethylbenzene to styrene. It was found that the conversion rate of ethylbenzene and the selectivity of styrene were as high as 92 μmol m-2 h-1 and 97.5% respectively, which was much higher than the previously reported metal-free catalysts. The active sites were found to be the surface C-Ti-O groups generated during the selective etching process and the two hydrogens on the ethyl group are stepwise abstracted from ethylbenzene as revealed by XPS analysis and first-principle calculations. The expanded layered structure facilitates the mass transfer and adsorption-desorption process. The stability performed in the long term test indicates its potential application in the dehydrogenation of hydrocarbons as an industrial catalyst.
This work entitled " Ti3C2Tx MXene Catalyzed Ethylbenzene Dehydrogenation: Active Sites and Mechanism Exploration from both Experimental and Theoretical Aspects" has been published online in ACS Catalysis.
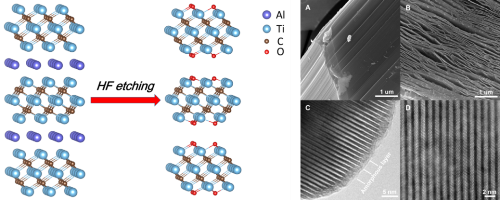
Fig. 1 Schematic representation of the preparation process and morphology of the Ti3C2Tx Mxene. (Image by IMR)
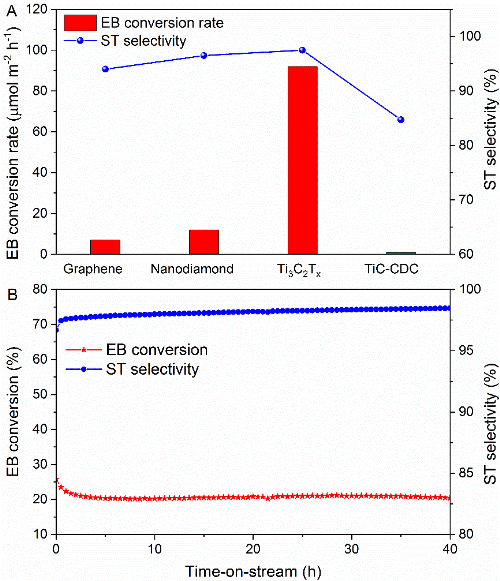
Fig. 2 A) Ethylbenzene dehydrogenation reactivity of Ti3C2Tx MXene, nanodiamond, graphene and TiC-derived carbon (TiC-CDC), B) Long term stability of Ti3C2Tx MXene on dehydrogenation of ethylbenzene.(Image by IMR)
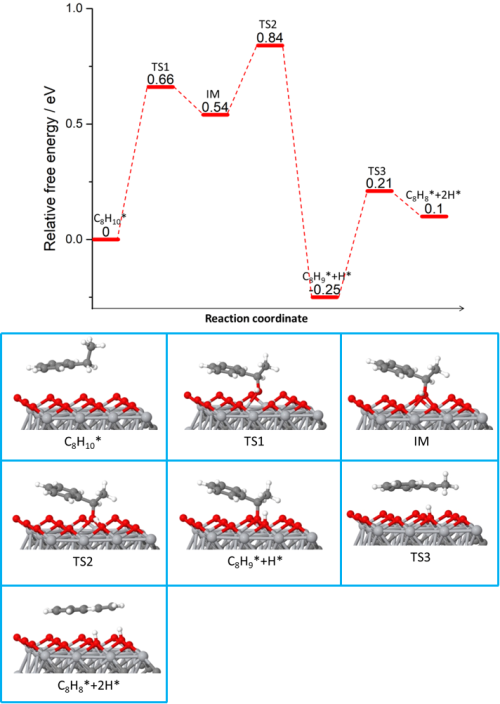
Fig. 3 The reaction pathway and important structures of ethylbenzene dehydrogenation on Ti3C2O2. Ti is light gray, C is dark gray, O is red and H is white.(Image by IMR)