Associate Prof. LIU Hongyang and his team from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) recently reported a Tin assisted fully exposed Pt clusters are fabricated on the core-shell nanodiamond@graphene (ND@G) hybrid support for dehydrogenation reaction. ACS Catalysis published this work online as a journal cover (DOI: 10.1021/acscatal.9b00601).
Light olefins, a widely used feedstock, are important building blocks for the synthesis of polymers and other value-added chemicals. Direct dehydrogenation (DDH) of light alkane is a typically industrial process for production of olefin which is an endothermic reaction thus requires high temperatures to obtain satisfactory conversion rates and olefin yields.
However, DDH could also lead to serious catalyst deactivation by sintering of active sites and coking at high reaction temperatures. To date, Pt3Sn alloy catalyst (Pt3Sn/Al2O3) is widely recognized as one of the best catalysts for this reaction, but rapid deactivation is still a main problem because the sintering of Pt3Sn nanoparticles (NPs) is unavoidable during dehydrogenation process. Meanwhile, only the surface Pt atoms in Pt3Sn NPs can participate in the catalytic reaction which is uneconomical for Pt utilization. Therefore, developing a better dispersed and more stabilized Pt-based catalyst is pivotal for the DDH of alkanes.
Associate Prof. LIU and his colleagues are dedicated to develop new nano-carbon material supported metal catalysts. They firstly reported the Pt-Sn catalyst with unique Pt species, fully exposed Pt clusters anchoring over the ND@G support (a-PtSn/ND@G). They use aberration-corrected transmission electron microscopy (Fig. 1) and X-ray absorption fine structure (XAFS) spectra (Fig. 2) constructed the structures of three Pt atoms anchored on ND@G. The as-synthesized a-PtSn/ND@G catalyst shows excellent catalytic performance in DDH of n-butane at a relatively low temperature (Fig. 3). Also, they built the catalyst structures and studied the reaction mechanism by quantum chemistry simulation at the DFT level (Fig. 4). The atomically dispersed Pt clusters has guaranteed a full metal availability to the reactants, a high thermal stability as well as an optimized adsorption/desorption behavior. This work paves the way for rational design of highly activity catalysts for dehydrogenation.
This work was supported by NSFC, the Ministry of Science and Technology, the Youth Innovation Promotion Association, CAS, Institute of Metal Research, SINOPEC and Shanghai Synchrotron Radiation Facility (SSRF).
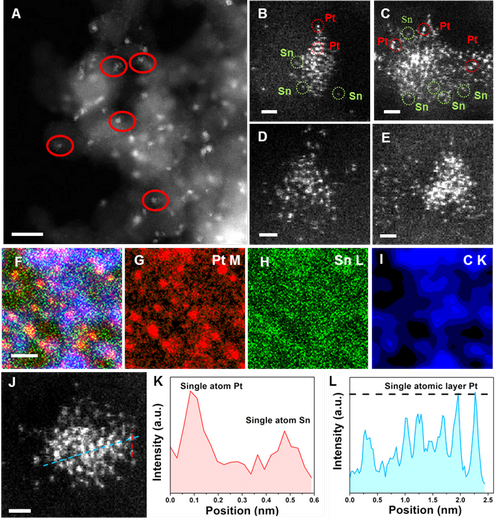
Figure 1. HAADF-STEM images of the a-PtSn/ND@G catalyst. (Image by IMR)
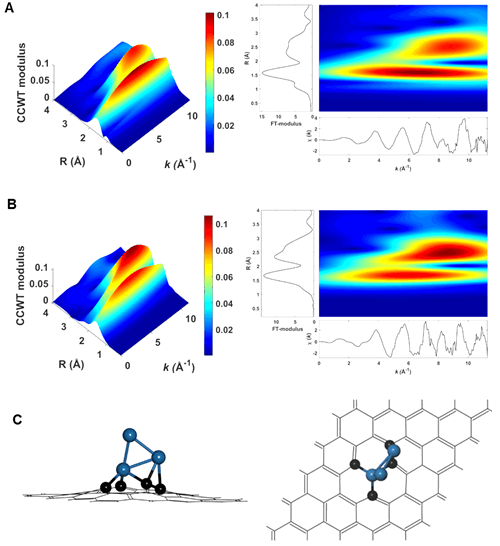
Figure 2. Wavelet transform (WT) analysis of different catalysts, A) a-PtSn/ND@G and B) Pt/ND@G. C) The optimized structure of Pt3 cluster embedded into graphene (Pt3-Gr, through Pt-C bond) from top and side view.(Image by IMR)
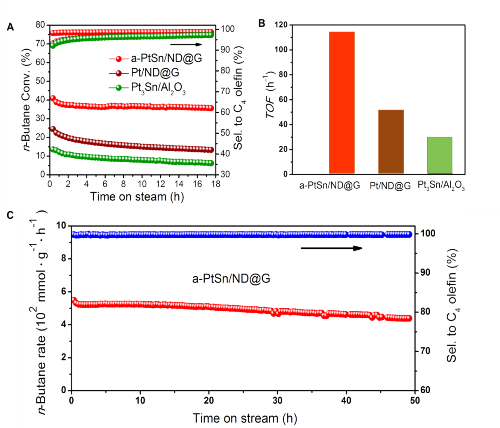
Figure 3. Direct dehydrogenation of n-butane over the different catalysts.(Image by IMR)
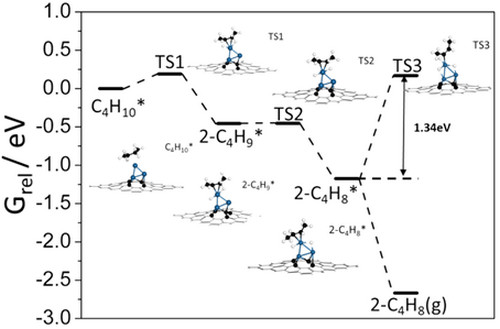
Figure 4. Gibbs free energy profile of direct butane dehydrogenation on the Pt3-Gr.(Image by IMR)
