ZSM-5 zeolite plays a vital role in petroleum cracking and refining of petrochemical products due to its microporous structure (0.51-0.56 nm) and tunable solid acid characteristics. Since zeolite micropores limit the mass transfer of reactants, in recent decades, the research focus has been to improve the accessibility of reactants to the acid sites of zeolites to improve their catalytic activity.
Usually, alkaline solution post-treatment is used to selectively desiliconize and etch out the intracrystalline mesopores to improve the accessibility of the acid sites of the zeolite. However, because the four-coordinated aluminum in the pristine zeolite inhibits desiliconization, this method is only suitable for the synthesis of ZSM-5 zeolites with high silicon to aluminum ratio, and the synthesis of low silicon to aluminum ratio ZSM-5 with good accessibility remains challenging.
Recently, researchers from Shenyang National Laboratory for Materials Science, the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) developed a novel and effective method to prepare hollow ZSM-5 zeolite catalyst with low silicon to aluminum ratio, collaborating with the University of Manchester. These findings were published online in Angewandte Chemie International Edtion, highly valued by editors as “very important”.
In this study, a pristine zeolite containing extra framework aluminum was prepared from a high-temperature rapid aging zeolite synthesis mother liquor. Then, the extra framework aluminum is reinserted into the frame aluminum by dissolution and recrystallization of tetrapropylammonium hydroxide, and a hollow structure is formed at the same time.
The novelty of this method lies in the use of pristine zeolite containing extra framework aluminum as the mother phase, which breaks the restriction of zeolite dissolution and recrystallization by the four-coordinated framework aluminum, thereby hollow ZSM-5 zeolite with silicon to aluminum ratio as low as 16 was prepared. Hollow ZSM-5 zeolite with low silicon to aluminum ratio shows excellent catalytic performance in hydrocarbon cracking, especially in the cracking of bulky molecules, with high activity, high stability and high propylene selectivity. The mass transfer characteristics of zeolite catalysts were studied by pulsed field gradient nuclear magnetic resonance (PFG-NMR).
It was shown that the excellent catalytic performance of low silicon to aluminum ratio hollow ZSM-5 zeolites originated from good accessible acid sites. This method is not only suitable for the synthesis of low silicon to aluminum hollow ZSM-5 zeolites, but also provides a feasible technical route for other hollow heteroatom-doped zeolites.
This research was supported by the China Scholarship Council, Shenyang National Laboratory for Materials Science and the EU Horizon 2020 project. Dr. JIAO Yilai is the first author. Co-corresponding authors are Dr. JIAO Yilai, Xiaolei Fan and Carmine D’ Agostino.
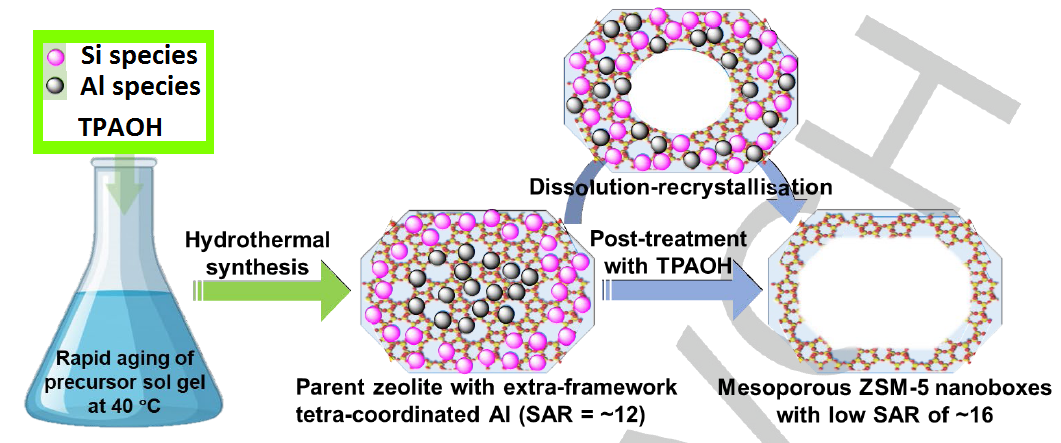
Figure 1. Schematic illustration of the preparation of the mesoporous ZSM-5-P nanoboxes via the rapid ageing (of the precursor sol gel mixture) and postsynthetic TPAOH treatment. (Image by IMR)
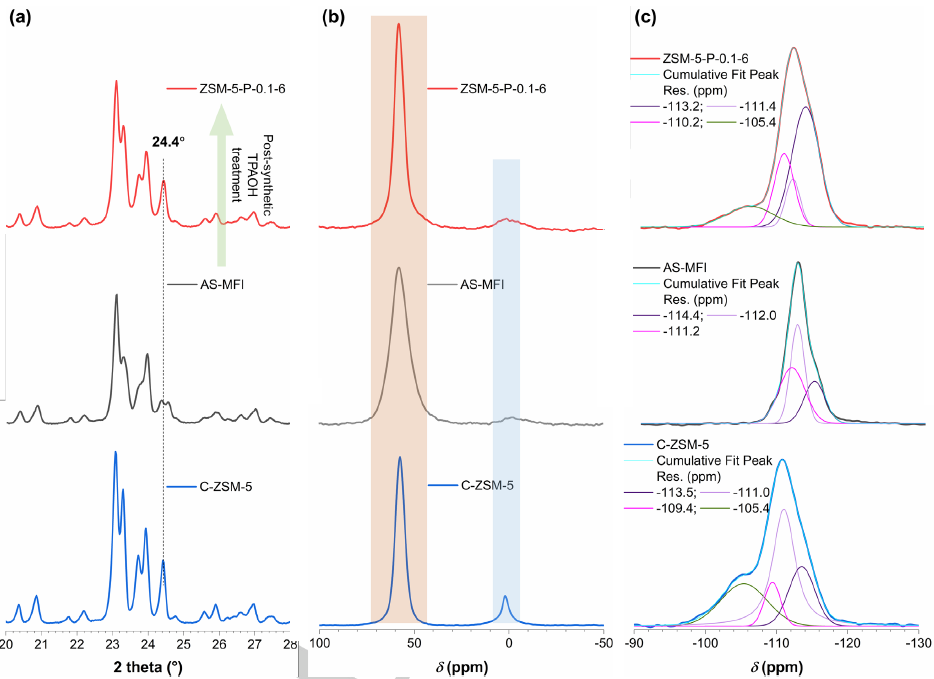
Figure 2. (a) XRD patterns, (b) 27Al MAS NMR spectra and (c) 29Si MAS NMR spectra of ZSM-5-P-0.1-6 (top), AS-MFI (middle) and C-ZSM-5 zeolites (bottom). (Image by IMR)
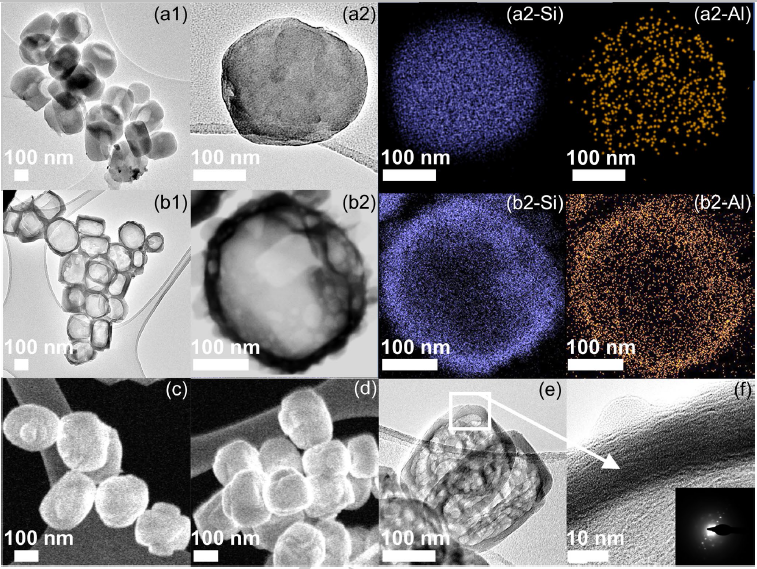
Figure 3. HRTEM micrographs of (a1–a2) AS-MFI and (b1–b2) ZSM-5-P-0.1-6 zeolites; STEM micrographs of (c) AS-MFI and (d) ZSM-5-P-0.1-6 zeolites; (e, f) HRTEM micrograph of ZSM-5-P-0.1-6 zeolite (Inset: the corresponding fast Fourier transform (FFT) of HRTEM). (Image by IMR)
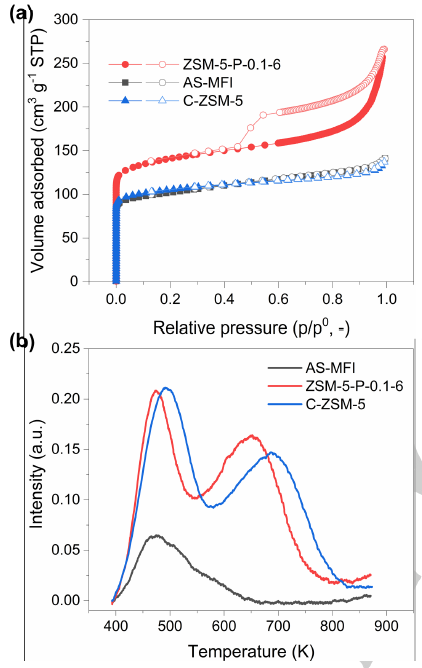
Figure 4. (a) N2 adsorption (solid symbols)/desorption (open symbols) isotherms and (b) NH3-TPD curves of AS-MFI, C-ZSM-5 and ZSM-5-P-0.1-6. (Image by IMR)
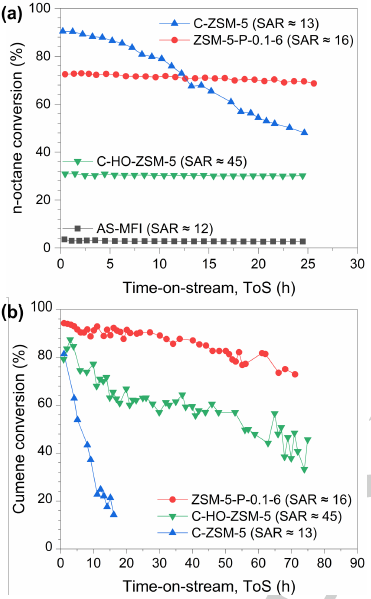
Figure 5. Conversion profiles of different zeolite catalysts as a function of time-on-stream (ToS) in (a) catalytic n-octane cracking and (b) catalytic cumene cracking. (Image by IMR)
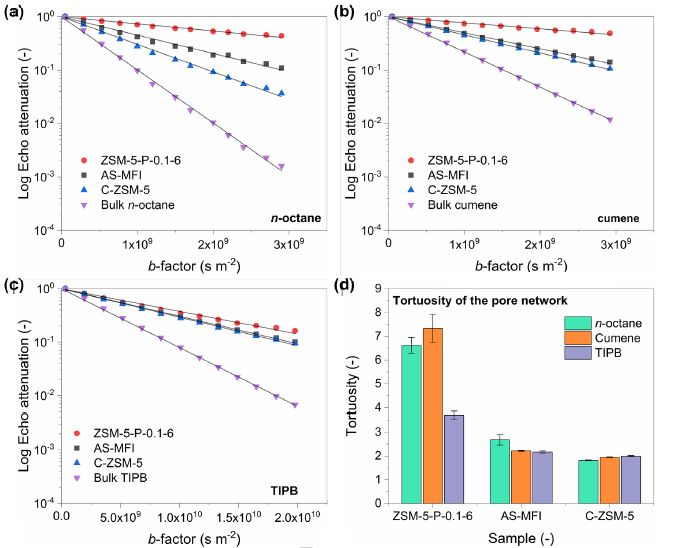
Figure 6. PFG-NMR log attenuation plots for (a) n-octane, (b) cumene and (c) TIPB within different zeolite samples under investigation (solid lines represent the fittings using Eq. S1); (d) values of tortuosity of the probe molecules of n-octane, cumene and TIPB in different zeolite samples. (Image by IMR)