Hydrogen peroxide (H2O2) is considered as one of the most important green oxidants for a wide range of industries, including wastewater treatment, papermaking and green chemical synthesis etc. On site production of H2O2 via two electron oxygen reduction reaction (2e-ORR) process has attracted enormous attention in the fields of green chemistry, chemical engineering, environmental and material science.
Systematic works on highly efficient hydrogen peroxide electrosynthesis with nano-carbon as catalytic materials has been conducted in Energy Catalysis & Materials group in Shenyang National Laboratory for Materials Science (SYNL), Institute of Metal Research (IMR).The research team led by Prof. QI Wei firstly revealed that the active sites for 2e-ORR could be attributed to carboxyl and carbonyl groups on nanocarbon catalysts via linking the catalytic activity with the accurate chemical composition and structure of nano-carbon materials. The detailed kinetic analysis indicated that the key for the selectivity of 2e/4e-electron product is the adsorption/desorption strength between the active sites of carboxylic groups and H2O2.
Following these fundamental understandings on the catalytic process and corresponding structure-function relations, Prof. QI Wei and co-workers have developed a novel carbon/surfactant composite electrode for highly selective hydrogen peroxide electrosynthesis with cooperations with research teams from Peking University and Fuzhou University.
The system was designed based on the principle of surface engineering and kinetic modulation without changing the surface chemical properties of the carbon catalyst, and the important role of the cationic surfactant is to weaken the interactions between carboxyl active sites and H2O2, thus promoting the efficiency and stability for H2O2 electrosynthesis. In alkaline media, a sustainably high selectivity of peroxide (>96%) across the potential window over 0.8 V for over 10 h could be achieved with the proposed carbon/surfactant system, which outperforms typical noble metal catalysts becoming the new record for H2O2 electrocatalysts.
These findings are anticipated to highlight the importance of interface design in electrocatalytic reaction systems and to provide mechanistic insight for advancing the potential practical applications of H2O2 electrosynthesis. These serial research works are published in Journal of Colloid and Interface Sciences and Chem. Miss LU Xingyu and Dr. WU Kuang-Hsu from Energy Catalysis & Materials group are first authors for these two papers, respectively. The authors acknowledge the financial support from NSFC, the Youth Innovation Promotion Association and SYNL.
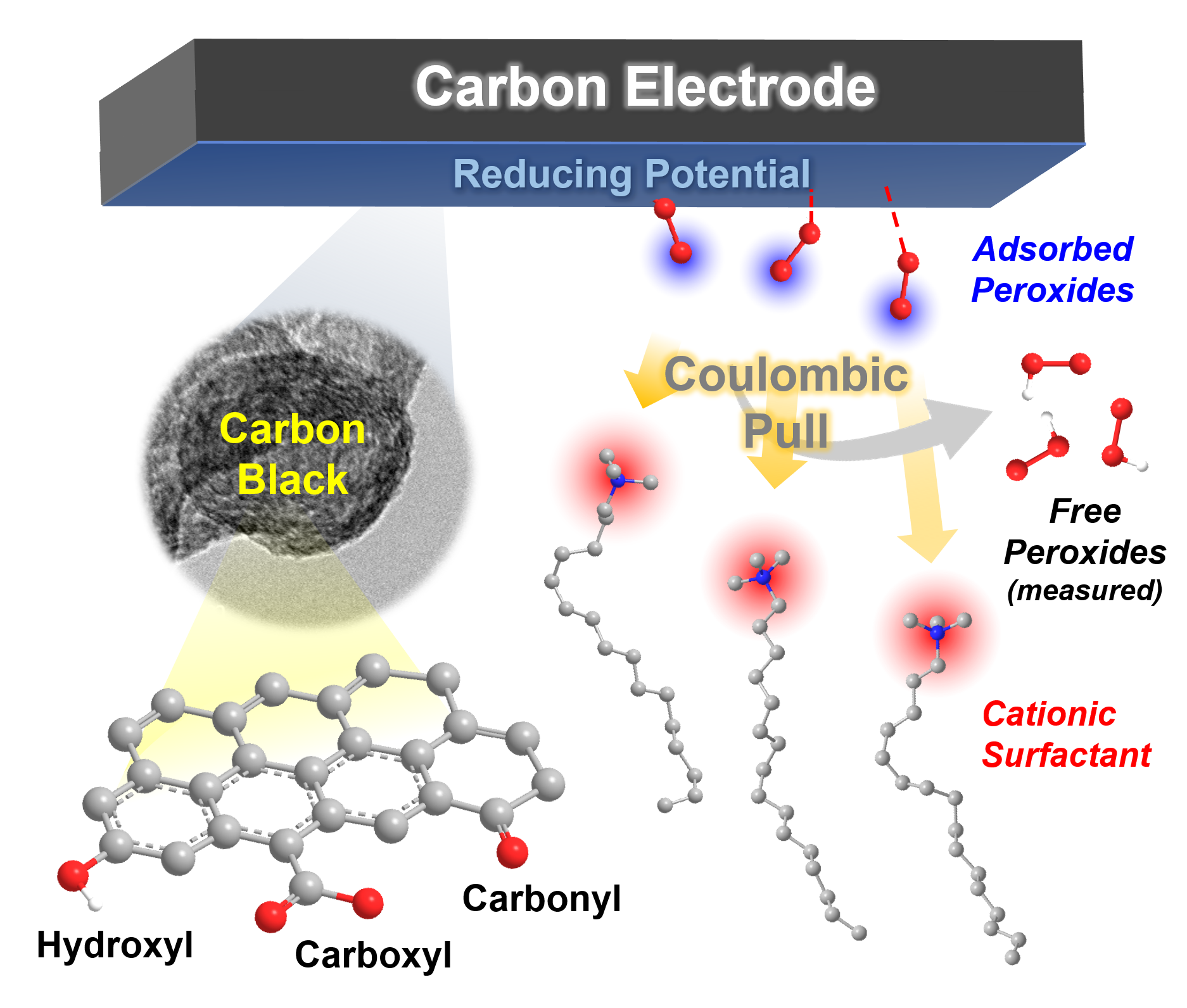
In-situ selectivity modulation by surface-acting cations at the carbon surface (Image by IMR)
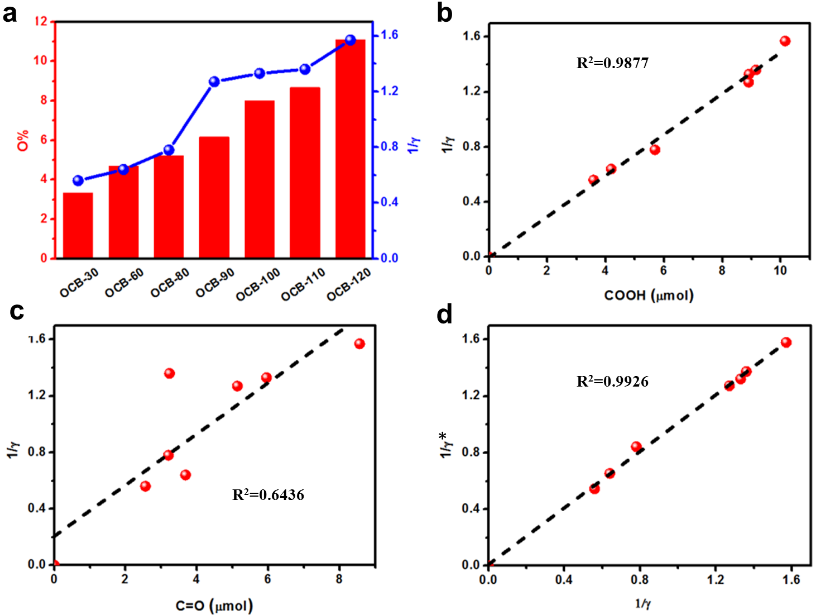
(a) Two-electron selectivity (1/γ) and oxygen content for different carbon materials: kinetic fitting (1/γ*) and experimental measurements (1/γ) (Image by IMR)
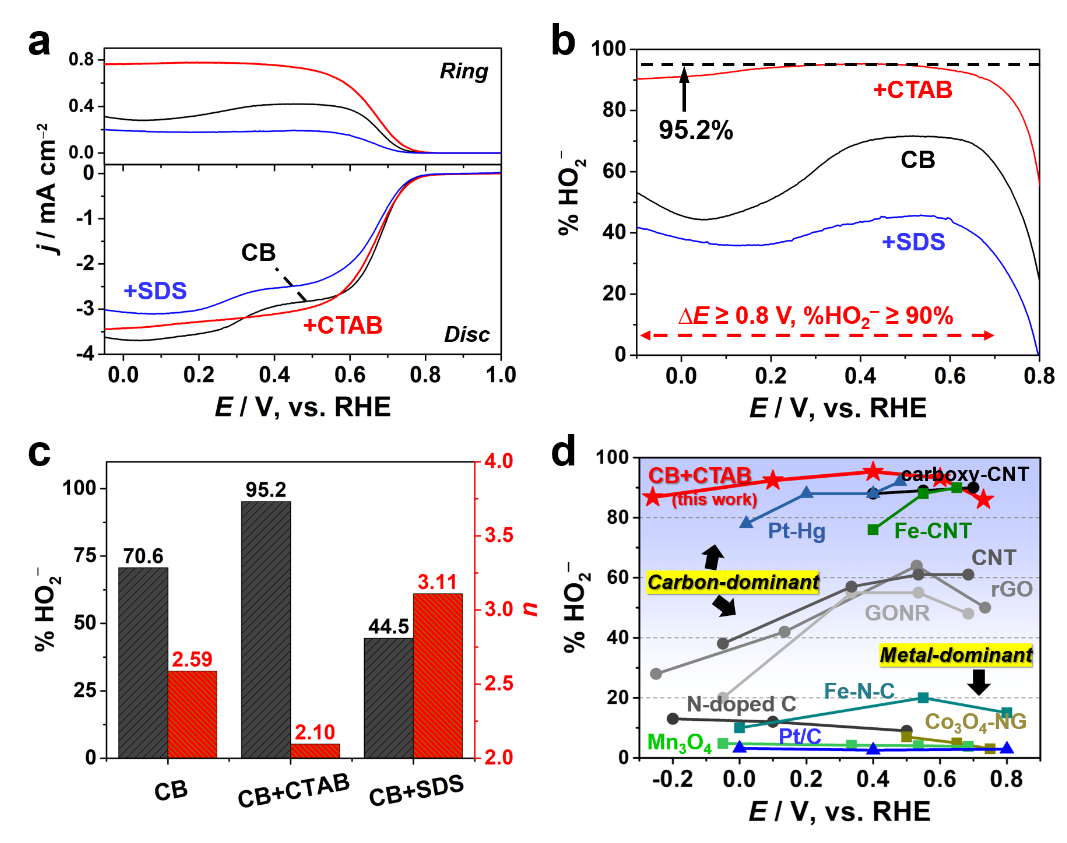
Selectivity performance of the proposed carbon/surfactant (CTAB) system: comparisons of peroxide selectivity and potential window width among reported electrocatalyst systems (Image by IMR)
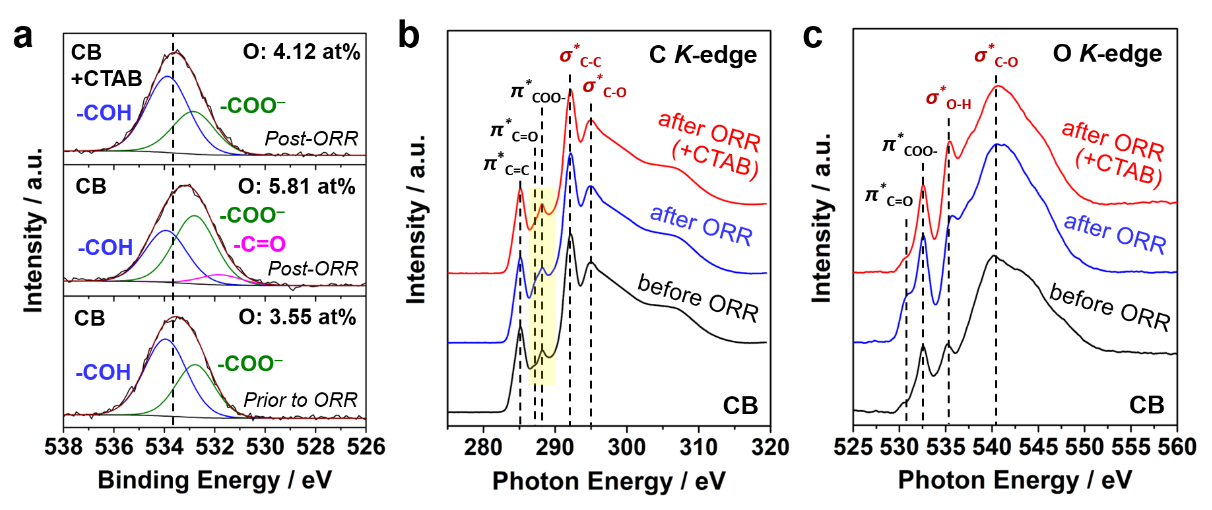
Surface oxygen chemistry of the proposed carbon/surfactant (CTAB) system: XPS O1s spectra and NEXAFS C and O K-edge spectra before and after ORR (Image by IMR)
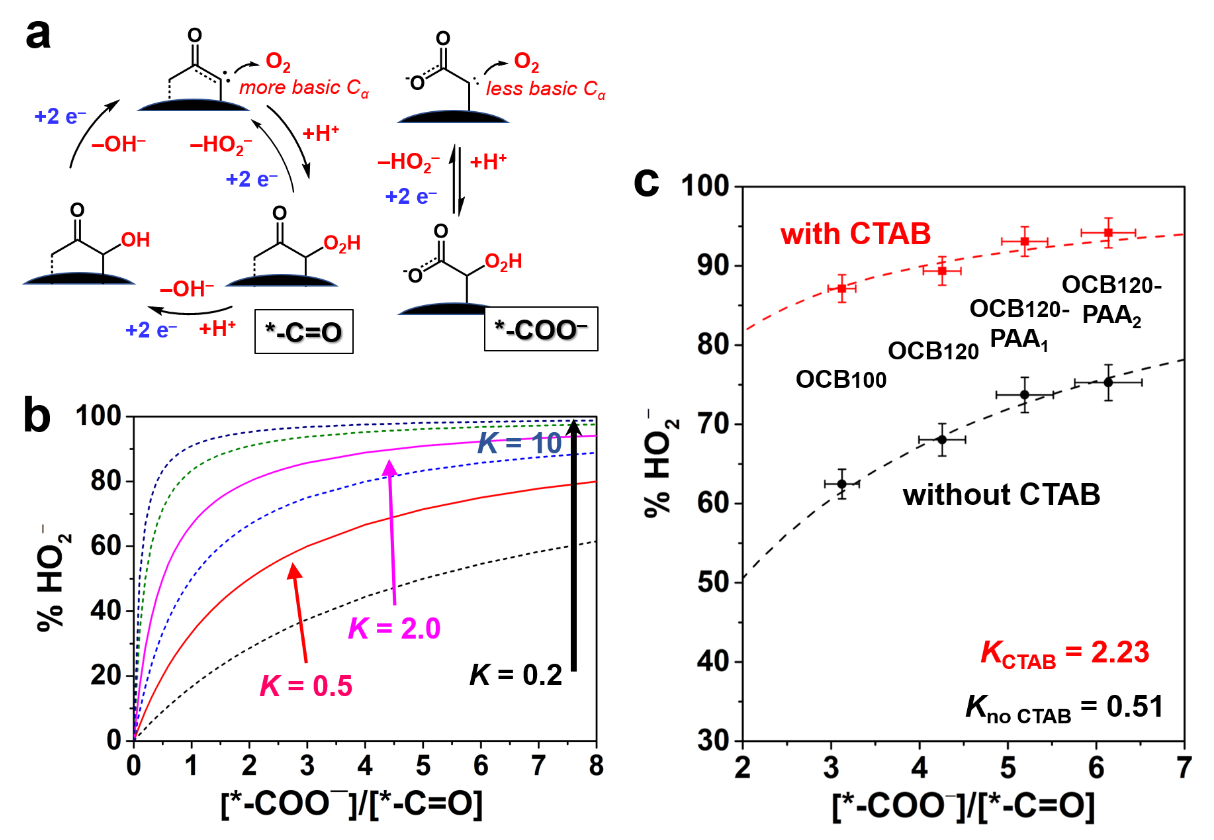
The kinetic model analysis of carbonyl and carboxyl functionalities (Image by IMR)