Conversion of renewable biomass resources into corresponding energy carriers or high value-added fine chemical precursors is an effective and important solution to solve the urgent problems in energy depletion and environmental pollution from modern society. The primary alcohols (methanol, ethanol or butanol etc.), which could be directly derived from biomass, served as key platform molecules that have great potential in the production of various types of fine chemicals. Efficient conversion of primary alcohols to corresponding high value-added compounds, such as aldehydes or ethers, have attracted enormous research interests in the field of chemistry, chemical engineering and material science. Conventional industrial catalyst systems applied in these processes are normally noble metal or transition metal species, which face serious intrinsic shortcomings, including limited reservation, high pollution and toxicity and energy consumption.
The Energy Catalysis & Material (ECM) group from Shenyang National Laboratory for Materials Science (SYNL), Institute of Metal Research (IMR) concentrated in the research topic for efficient utilization and substitution of metal-based catalysts, especially in the reaction mechanism and kinetics of nanocarbon catalysis.
Recently, scientists from ECM have developed novel carbon-based catalyst systems for efficient chemical conversion of primary alcohols to high value-added products. The research team firstly discovered the reaction routes of carbon nanotube catalyzed methanol conversion process via active site titration, reaction kinetics and model catalyst analysis, which mainly includes the formation of dimethyl ether and formaldehyde via acid and redox pathways, respectively. Carboxylic acid and ketonic carbonyl groups on carbon nanotubes serve as the active sites for these reaction pathways (Catalysis Science & Technology).
The applications of carbon catalysts in n-butanol conversion systems exhibited the same reaction properties, and the structure-function relation analysis revealed that the conjugation size and the doped heteroatoms had shown significant effect on the intrinsic catalytic activity of nanocarbon. Rational adjusting the chemical/electronic structure and the redox/acid catalytic activity of nanocarbon may effectively tune the reaction pathway and lead to the desired product distribution (Carbon).
Based on these basic understandings on the reaction mechanism and structure-function relations, Prof. QI Wei from ECM group worked together with Prof. LIN Sen and Prof. XIE Zailai from Fuzhou University, realizing the rational design and fabrication of novel graphene/boron nitride (BCN) composite catalysts for highly efficient primary alcohol conversion process. One of the unique properties of BCN is the hybridization of carbon (graphitic domain) and boron nitride species in nanoscale, which effectively promotes the thermal stability of the composite catalysts especially under the oxidation atmosphere. The combination of the two components also enhanced the catalytic property in the maximal extent via synergistic effect. The experiment and theoretical calculation results revealed the detailed reaction mechanism at molecular (atomic) level (Science Advances).
These serial works were published as full papers this month, and the first authors were the graduate students from ECM: Pengqiang Yan, Fan Li and Xuefei Zhang. The Chinese patents on related carbon materials and reaction systems were also issued, which may shed light on the potential industrial applications of carbon-based catalytic materials. The authors sincerely acknowledge the financial support from NSFC, China, the Youth Innovation Promotion Association, CAS, China and SYNL.

Fig. 1, Schematic drawings for the synthesis procedure of BCN nanotubes and methanol conversion process on BCN catalysts (Image by IMR)
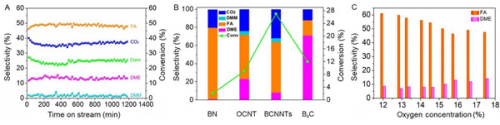
Fig. 2, Activity, target product selectivity and stability of BCN nanotube catalysts for methanol conversion reactions (Image by IMR)

Fig. 3, Identification and quantification of active sites for dimethyl ether (DME) and formaldehyde (FA) on BCN nanotube catalysts (Image by IMR)

Fig. 4, Reaction mechanism for methanol conversion on BCN catalysts: evolution of -B-OH sites during reactions (Image by IMR)
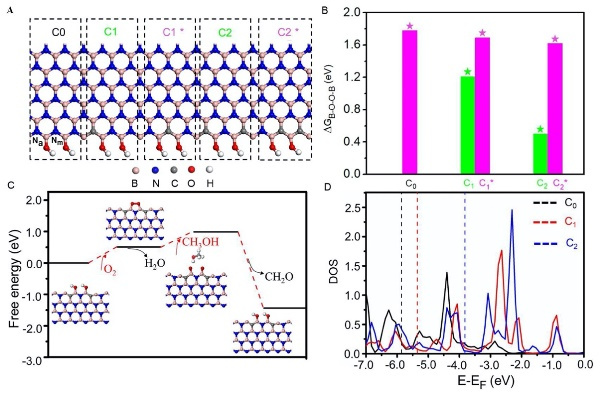
Fig. 5, Active sites and reaction mechanism for BCN catalyzed methanol conversion reactions: theoretical calculation results (Image by IMR)