Light olefins are the backbone of the chemical industry and the basic raw materials for synthetic plastics, rubber and fibers. Olefin production is one of the important indicators to measure technical level of a country's chemical industry. With economic development, the demand for olefins continues to increase. China's olefins consumption is account for more than 15% of the global consumption in 2020. Therefore, further improvement of olefin production efficiency has important economic value and social significance. On the other hand, the dehydrogenation of alkanes can efficiently convert light alkanes into olefinic counterpart. At present, the (direct and oxidation) dehydrogenation of alkanes are facing challenges such as low selectivity, carbon deposition, and high energy consumption. The design and development of new efficient catalysts is the key to solving these problems. In addition, revealing the catalyst properties and reaction mechanism at the molecular level is a prerequisite for targeted screening of high-efficiency catalysts.
Since 2014, the research team of associate Prof. LI Bo of the Joint Research Department has been working on first principles calculation simulation on the dehydrogenation of light alkanes. Based on the researches, the unique catalytic performance of the carbon-based catalyst sp2/sp3 core-shell structure in the dehydrogenation of alkanes was revealed (ACS Catalysis, 7, 3779-3785 (2017)), and the detailed description of the carbon deposition process of the industrial platinum-based catalyst and the method of controlling the carbon deposition were given (ACS Catalysis, 8, 4694-4704 (2018)).
Recently, the research team systematically studied the reaction mechanism of oxidative dehydrogenation of ethylbenzene on nanocarbon catalyst by first-principles-based micro kinetic modeling. The results show that the reaction mainly occurs through the ER pathway, and the free radical reaction yields a significant contribution to the total reaction rate under certain conditions. This study provides a decisive description of the mechanism of ODH on nanocarbon catalysts under various reaction conditions and sheds light on design of the catalyst and optimization of reaction conditions. This work was recently published in ACS Catalysis (doi: 10.1021/acscatal.0c02952).
This work combines first principles calculations and micro-kinetic modeling to study the reaction mechanism of ethylbenzene oxidative dehydrogenation on nanocarbon catalyst and its changes under different reaction conditions. Three possible mechanisms of active site regeneration are considered. In addition, the reaction process between weakly adsorbed species and free radicals was discovered innovatively. The reaction energy and energy barrier of the elementary reactions are obtained from the first-principles calculations and used as the input of the micro-kinetic modeling. First principles calculations and micro-kinetic modeling show that both the ketone carbonyl group and the weakly adsorbed HO2. radical have catalytic activity for the dehydrogenation of alkanes. The ER pathway is more likely than both LH and MvK pathways under various conditions. In addition, the reaction can also occur through weak adsorbed HO2. under certain conditions. The reaction is first-order in ethylbenzene and zero-order in O2 when the ratio of reactants nears stoichiometry. The reaction rate scales linearly with the partial pressure of EB and does not change with the partial pressure of O2 in this region. The defect sites in the catalyst are often deemed as intrinsically high active centers, and the calculation results also confirm their high activity, but the density of the defect sites in the initial catalyst does not affect the intrinsic activity of the catalyst and the reaction state at steady state. Current work not only deepens the understanding of the mechanism of oxidative dehydrogenation of alkanes on nanocarbons, but also identifies the unique properties of non-metallic catalysts.
This work was supported by the National Natural Science Foundation of China,National Laboratory for Materials Science,SINOPEC. The computations were also supported by the Special Program for Applied Research on Super Computation of the NSFC Guangdong Joint Fund (the second phase).
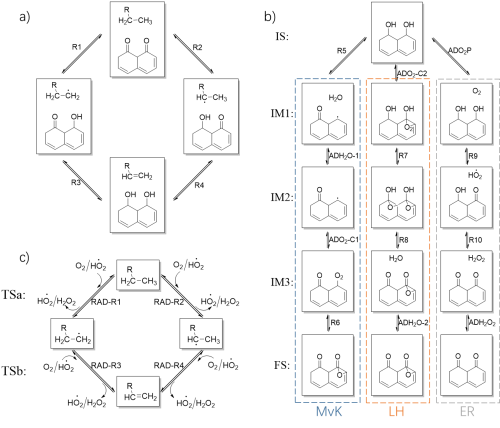
Figure 1. Network of elementary reactions of ODH. (a) The dehydrogenation of EB. (b) The regeneration of the catalyst. (c) The reactions between weakly adsorbed species and radical species. (Image by IMR)
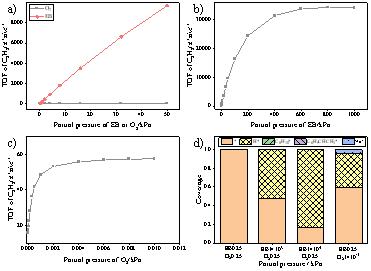
Figure 2. Rate of production of C8H8 and main species at the steady state at different EB/O2 partial pressures. (a-c) The TOF of C8H8 under different partial pressures of EB and O2. (d) The coverage of species under different partial pressures. (Image by IMR)
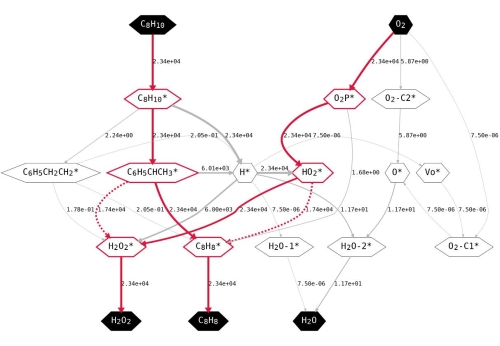
Figure 3. The network and rate of species transformation under 800 K, 1 bar EB, 1 bar O2. The key intermediates and pathways are highlighted in red. The dashed lines indicate the contribution of radical reactions. The numbers on the right side of connecting lines represent the calculated rate of species transformation. (Image by IMR)