Lithium (Li) metal anode, with the highest specific capacity (3860 mAh g-1) and the lowest redox potential (-3.04 V vs standard hydrogen electrode), is considered as potential alternative for the high-energy-density next-generation lithium batteries. However, the unstable electrolyte-Li metal anode interface has been the biggest obstacle for the practical application of the batteries with Li metal anode.
The research teams led by Prof. BAI Shuo and Prof. LI Feng from Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), and Prof. TAN Jun from Ji Hua Laboratory achieve spatially selective distribution of the targeted solvation structure of ions at the electrolyte-anode interface by fabricating micro-arrays of nano-hydroxyapatite (nHA) with high Li+ binding energy on copper (Cu) foil. This work was published in Advanced Materials.
The key point for a stable Li metal anode is to construct a robust solid electrolyte interface (SEI) film at the electrolyte-anode interface. The most ideal approach is to optimize the solvated structure of ions in the electrolyte especially at the electrolyte-anode interface but maintain the properties of the bulk electrolyte.
This study reveals that the electronegative nHA particles with high Li+ binding energy can effectively tune the solvation structure of ions in the electrolyte. Li+ will preferentially migrate to the surface of the nHA particle, forming a local Li+-rich region around the nHA particle, where anions can interact with more Li+ generating multi-coordinated anions. Based on this finding, micro-arrays of nHA are further prepared on Cu foil (current collector of the anode) to preferentially form multi-coordinated anions at the electrolyte-anode interface. Meanwhile, the experiment also verifies that the micro-arrays do not affect the solvation structure of the bulk electrolyte.
Generally, uncoordinated anions will be strongly repulsed by the electron-rich anode, which largely reduces the decomposition efficiency of the anions. In this study, with nHA micro-arrays, the multi-coordinated anions at the electrolyte-anode interface can be carried by Li+ to effectively cross the electric double layer on the anode, which is desired for an anion-derived SEI film. The anions in the electrolyte more completely decompose into highly protective inorganic components in the SEI film, which can effectively suppress the dendrite growth on the anode. As a result, at high charge-discharge current density, the risk of the notorious micro-short circuit happening in Li metal batteries is significantly reduced. The finding of electronegative materials tuning local solvation structure in the electrolyte provides new design principles to build robust SEI for stable Li metal batteries.
This study was done in collaboration with YU Tong and TAI Kaiping from IMR.
The work was supported by National Natural Science Foundation of China, National Key R&D Program of China, Strategic Priority Research Program of the Chinese, Key R&D Plan of Jihua Laboratory, China National Postdoctoral Program for Innovative Talents, and China Postdoctoral Science Foundation.
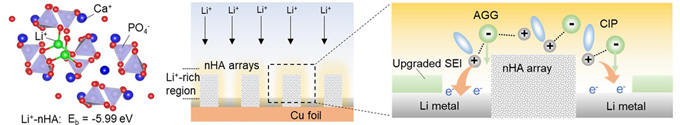
Interaction of nano-hydroxyapatite with lithium ions and effect on the formation of solid electrolyte interface (Image by IMR)