The electrocatalytic nitrate reduction reaction to ammonia (eNO₃⁻RR-to-NH₃) offers a promising avenue for transforming nitrate-containing wastewater into valuable ammonia, thereby enabling sustainable wastewater valorization.
To address the challenges of suboptimal ammonia selectivity and competitive hydrogen evolution in iron-based electrocatalysts for eNO₃⁻RR-to-NH₃ process, a research team led by Prof. Gang Liu from the Institute of Metal Research (IMR) of the Chinese Academy of Sciences has developed a self-triggered localized alkalization strategy for the Co₄Fe₆ electrocatalyst. The innovative strategy significantly boosts the performance of Co₄Fe₆ in the eNO₃⁻RR-to-NH₃ process, thereby improving both NH₃ selectivity and overall reaction efficiency.
This work was published in Journal of the American Chemical Society.
The research team synthesized the Co₄Fe₆ electrocatalyst through a facile solvothermal method coupled with low-temperature thermal reduction. The electrochemical performance results revealed that the Co₄Fe₆ alloy achieves excellent nitrate-to-ammonia selectivity (>99%) across a wide range of electrolyte concentrations and potentials, significantly outperforming monometallic Fe and Co counterparts. Notably, a membrane electrode assembly (MEA) electrolyzer incorporating Co₄Fe₆ demonstrated continuous operation over 500 hours at industrial-level current densities, enabling simultaneous high-efficiency ammonia synthesis and solid-state ammonium salt recovery.
The exceptional performance originates from two synergistic mechanisms: (1) The in situ generation of Fe2+ad species that spontaneously induce localized alkalinity enhancement, thereby bypassing the conventional pH-dependent kinetic limitations; (2) The Co-Fe dual active sites synergistically accelerate the water dissociation and optimize the adsorption of reaction intermediates.
"We have developed a novel self-triggered localized alkalization strategy that enables alkaline-level catalytic performance in neutral media," said Prof. Liu. "This innovation effectively suppresses hydrogen evolution side reactions, thereby maintaining exceptional selectivity and Faradaic efficiency for ammonia production."
This work achieves dual breakthroughs in both material design and mechanistic understanding for iron-based eNO₃⁻RR electrocatalysts, while advancing sustainable nitrate upcycling technologies toward industrial deployment. The findings provide fundamental insights and practical guidelines for developing next-generation energy conversion materials.
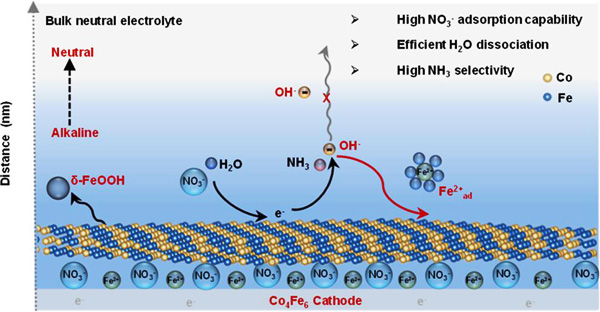
Local pH enhancement mechanism for the Co₄Fe₆ cathode (Image by IMR)
Key Words:
eNO₃⁻RR-to-NH₃; electrocatalyst; localized alkalinity; metal alloy;
原文链接