Researchers from the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS) have unveiled an innovative strategy that overcomes the critical challenge of catalyst stability in biomass electrooxidation. This breakthrough paves the way for achieving industrial-level current densities to co-produce hydrogen and fine chemicals from biomass valorization, which have been published in Nature Sustainability entitled "Efficient glycerol electrooxidation at an industrial-level current density".
The study focuses on the glycerol oxidation reaction (GOR), a key pathway for converting biomass into valuable chemicals. Unlike traditional thermal catalytic methods requiring harsh conditions and toxic oxidants, creating environmental concerns, electrocatalytic GOR, uses water as the oxidant and renewable electricity as energy input, offering a sustainable alternative. More importantly, it can replace the energy-intensive oxygen evolution reaction in water electrolysis, enabling simultaneous production of green hydrogen and valuable chemicals.
However, a persistant challenge has been the instability of common GOR catalysts like transition metal oxides (Co₃O₄). The surface of Co₃O₄ catalyst would transform into amorphous oxyhydroxide phase under the industrial level current densities (>500 mA cm⁻²), leading to competing oxygen evolution reaction and low efficiency. This has severely limited practical applications of this reaction system.
The research team ingeniously solved this problem by introducing trace amounts of Cu²⁺ ions (only 1% of reactant concentration) into the electrolyte. They found that the reversible redox cycling between Cu²⁺ and Cu⁺ during electrocatalysis helps maintain the catalyst’s crystalline structure.
This simple yet effective method dramatically boosted the performance of Co₃O₄ catalysts. At a high current density of 800 mA cm⁻², the Faradaic efficiency for formic acid production increased dramatically from 62.2% to 99.3%. The system also showed excellent scalability and demonstrated a formic acid production rate of 13.2 g h⁻¹ on a 6×6 cm² electrode with over 100 hours of stable operation.
This approach represents a significant advancement toward practical biomass electrorefining. The method is also applicable to other transition metal oxides and various biomass electrooxidation reactions, offering new possibilities for green hydrogen production coupled with biomass valorization.
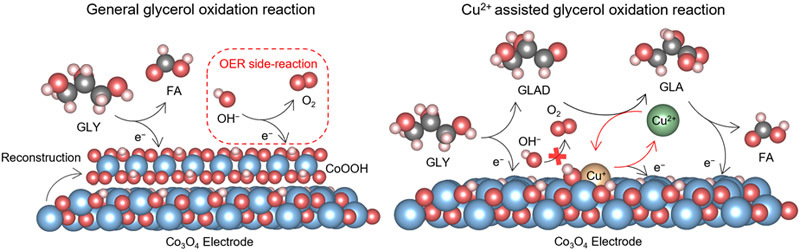
Schematic diagram illustrating the process of Cu²⁺ suppressing surface reconstruction of the catalytic material. (Image by IMR)