A collaborative research team led by Prof. LIU HongYang and Special Research Assistant Dr. CHEN Xiaowen from the Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, together with teams led by Prof. MA Ding from Peking University, Dr. CAI XiangBin from Nanyang Technological University, Prof. HE YuRong from Ningxia University, and others, has successfully constructed an Ir1Cu1 dual-atom catalyst on defect-rich graphene, achieving highly efficient low-temperature dehydrogenation of butane to butene. Furthermore, they discovered a reversible "aggregation-redispersion" structural evolution of the Ir1Cu1 dual-atom catalyst induced by switching between reaction gas and oxygen atmospheres. This effectively addresses the technical bottleneck of easy sintering and deactivation commonly faced by atomically dispersed metal catalysts in high-temperature reactions. This significant research achievement was recently published online in Nature Catalysis.
Olefins are crucial feedstocks for producing high-value-added chemicals in modern chemical industry, making their efficient preparation highly significant. Alkane dehydrogenation is an important direct route to produce olefins and has garnered widespread attention in both industrial production and fundamental research. However, how to reduce the energy consumption of dehydrogenation processes while efficiently utilizing hydrocarbon resources remains a critical technical challenge in the field. Due to the stable geometric configurations and thermodynamically strong chemical bonds of alkanes (such as ethane, propane, and butane), high temperatures (>550 °C) are typically required to overcome the reaction energy barrier for C-H bond cleavage. These high-temperature conditions easily trigger side reactions such as C-C bond cleavage, hydrogenolysis, and polymerization, as well as sintering of metal catalysts. This not only reduces olefin selectivity and shortens catalyst lifespan but also highlights the persistent need for improved intrinsic C-H bond activation capability of the catalysts. Therefore, designing low-temperature, high-efficiency dehydrogenation catalysts is a crucial research direction in alkane dehydrogenation.
In previous studies, Dr. CHEN XiaoWen discovered that single-atom Ir catalysts exhibit weaker adsorption capability for olefins, facilitating olefin desorption. This property not only leads to high olefin selectivity but also effectively suppresses deep dehydrogenation and demonstrates excellent anti-coking ability, showing great potential in alkane dehydrogenation reactions (Nat. Commun. 2023, *14*, 2588; Nat. Commun. 2021, *12*, 2664). However, the weak adsorption of alkanes and intermediates on Ir single atoms results in insufficient C-H bond activation capability, particularly for the second C-H bond cleavage. Building upon this foundation, the researchers introduced a Cu atom adjacent to the Ir single atom, constructing an Ir1Cu1 atomic pair catalyst. In butane dehydrogenation, the intrinsic activity of the Ir1Cu1 atomic pair reached as high as 2.45 s⁻¹ at 450 °C, which is 6.3 times that of the Ir single-atom catalyst, with C4 olefin selectivity exceeding 99%. Results from reaction kinetics experiments, temperature-programmed surface reaction studies, and DFT calculations indicate that the Ir1Cu1 dual-atom structure significantly reduces steric hindrance, providing more possibilities for the adsorption configurations of reactants and intermediates. Meanwhile, the neighboring Cu atom modulates the surface electronic state of the active Ir center, endowing the Ir atom with electron-rich characteristics, which favor bonding with C atoms and thus promote the adsorption of butane and its intermediates. Consequently, the Ir1Cu1 dual-atom site significantly lowers the energy barrier for C-H bond activation, shifting the rate-determining step from C-H bond cleavage to butene desorption, enabling low-temperature and efficient dehydrogenation of butane. The researchers extended the reaction system to propane and ethane dehydrogenation. In propane dehydrogenation, the intrinsic activity of the Ir1Cu1 dual-atom reached 3.19 s⁻¹ at a low temperature of 480 °C, while in ethane dehydrogenation, it reached 4.43 s⁻¹ at 530 °C. The results from butane, propane, and ethane dehydrogenation tests demonstrate that, when achieving comparable intrinsic activity, the dehydrogenation temperature for the Ir1Cu1 dual-atom catalyst is significantly lowered by approximately 150 °C compared to reported supported metal catalysts.
The research team subjected the Ir1Cu1 dual-atom catalyst after 10 hours of reaction to oxidative regeneration treatment and found that the dehydrogenation performance of the IrCu catalyst could be fully restored. For the first time, they discovered that the calcination atmosphere could induce structural evolution of the IrCu metal species. During regeneration, the Ir and Cu components, which had aggregated into Ir clusters and Cu clusters, redispersed into Ir atoms and Cu atoms, reforming the Ir1Cu1 dual-atom structure consistent with that before the reaction. Therefore, on the IrCu catalyst, the regeneration treatment not only removes coke deposits but also reconstructs the geometric structure and microenvironment of the Ir1Cu1 atomic pairs. This reversible "aggregation-redispersion" mechanism is applicable to the "deactivation-regeneration" process in alkane dehydrogenation reactions, further extending the catalytic lifetime. After the fourth regeneration cycle (total 40 h reaction), a significant number of Ir1Cu1 atomic pairs and single Cu atoms could still be observed on the IrCu catalyst, identical to the catalyst structure before reaction.
This work, through the innovative construction of an Ir1Cu1 dual-atom catalyst and the utilization of a neighboring Cu atom to modulate the microenvironment of the active Ir center, significantly enhances the catalyst's C-H bond activation capability. This drives the shift of the rate-determining step from C-H bond cleavage to butene desorption, achieving low-temperature efficient conversion of butane, propane, and ethane. It effectively addresses the key issues of high energy consumption and low activity in current dehydrogenation processes. Simultaneously, the Ir1Cu1 dual-atom catalyst demonstrates high durability during continuous regeneration cycles, providing a breakthrough solution to the problems of sintering and easy deactivation of atomically dispersed metal catalysts under high-temperature conditions. This holds significant importance and application value for designing efficient and regenerable low-temperature alkane dehydrogenation catalysts.
The above work received support from the National Natural Science Foundation of China, the National Key R&D Program, the National High-Level Talent Program, the China Postdoctoral Science Foundation, the Institutional Research Project of Large Scientific Facilities of the Chinese Academy of Sciences, as well as collaborative projects with enterprises including Sinopec, Sinochem, and CHN Energy. It also received strong support from the Shanghai Synchrotron Radiation Facility and the Beijing Synchrotron Radiation Facility.
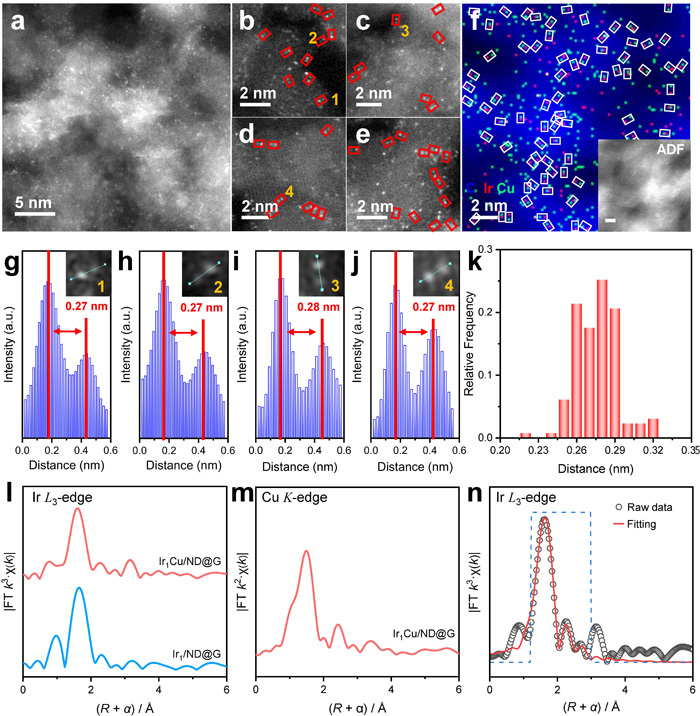
Structural Analysis of Ir1Cu/ND@G: (a)-(e) HAADF-STEM images, (f) EDX analysis, (g)-(j) line-scan intensity profiles, (k) statistical analysis of interatomic distances, (l) Ir L₃-edge k³-weighted EXAFS, (m) Cu K-edge k²-weighted EXAFS, (n) fitting results of the Ir L₃-edge k³-weighted EXAFS.(Image by IMR)
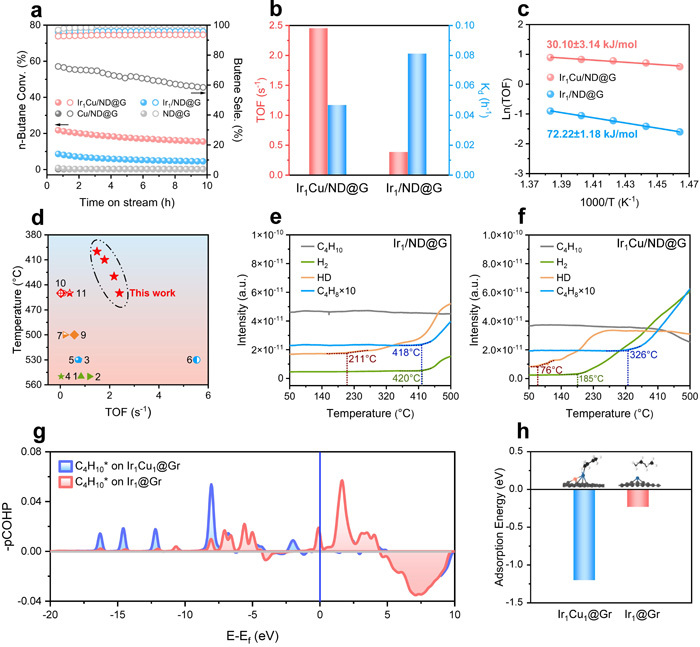
Butane Dehydrogenation Performance of the Catalyst: (a) Butane conversion as a function of time, (b) Comparison of the catalyst's TOF and k_d, (c) Results of apparent activation energy determination, (d) Comparison of dehydrogenation performance with other reported catalysts (for butane dehydrogenation), (e)-(f) TPSR results for butane dehydrogenation, (g) Calculated results of COHP, (h) Calculated results of butane adsorption energy. (Image by IMR)
Schematic Illustration of the Butane Dehydrogenation Process and the Reaction-Regeneration Cycle over the Ir1Cu1 Dual-Atom Catalyst (Image by IMR)