Researchers from the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), in collaboration with international partners, have made a breakthrough in understanding how electrochemical reactions drive topological defect evolution in battery materials at the atomic scale.
Using in-situ transmission electron microscopy, the research team led by Prof. WANG Chunyang, along with Prof. XIN Huolin from the University of California, Irvine and Prof. LI Ju from MIT, directly observed for the first time the nucleation, movement, and annihilation of dislocations triggered during battery operation.
The study, published in Proceedings of the National Academy of Sciences, focuses on layered oxide cathode materials-widely used in electric vehicles, which undergo complex phase transformations and chemomechanical degradations during electrochemical cycling.
"Unlike traditional structural materials where mechanical stress drives dislocation motion, here electrochemical reactions induce non-uniform lattice distortion, generating dislocations that significantly affect structural stability and battery performance," explained the research team.
A surprising finding was that dislocation climb and slip velocities driven by electrochemical reactions are comparable, differing from conventional materials where slip is typically orders of magnitude faster than climb under stress. The research also revealed novel mechanisms including simultaneous nucleation of cracks and rock-salt phases at dislocation cores, and local orientation changes caused by dislocation burst-annihilation events.
These atomic-scale insights into topological defect dynamics not only expand fundamental understanding of defect behavior under multi-field coupling but also provide important theoretical guidance for developing next-generation battery materials with enhanced structural stability and longevity.
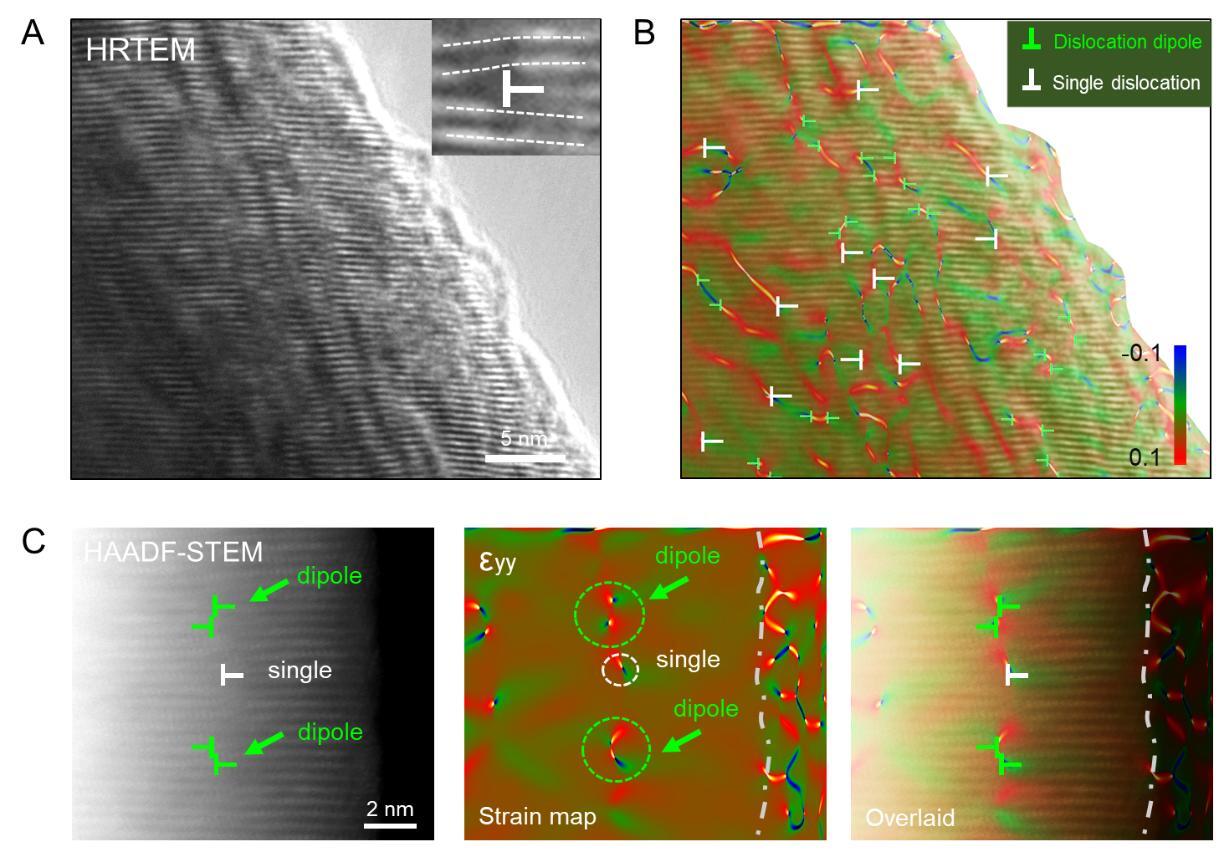
Electrochemically triggered dislocation nucleation in layered oxide cathode materials (Image by IMR)
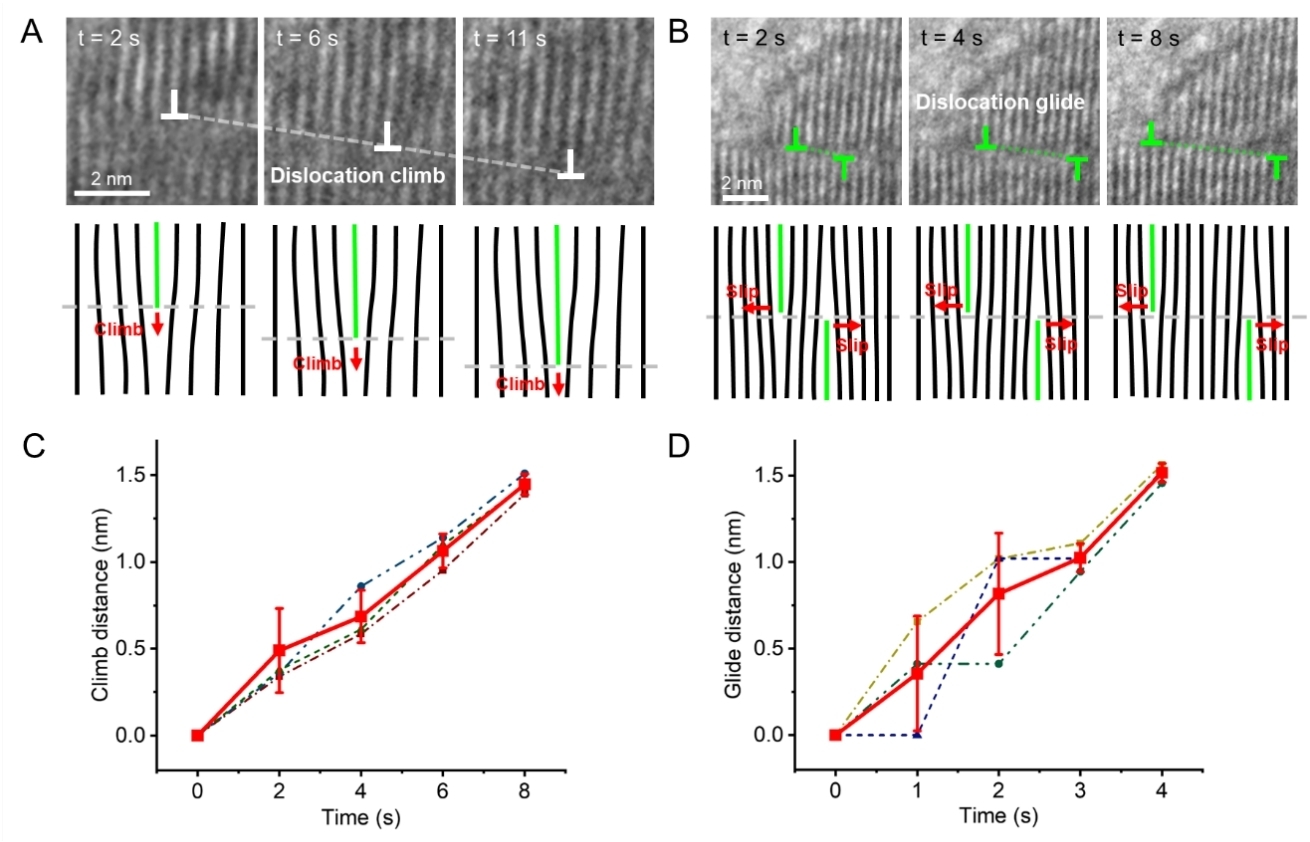
Measurement of the dislocation slip/climb velocities during in-situ delithiation (Image by IMR)
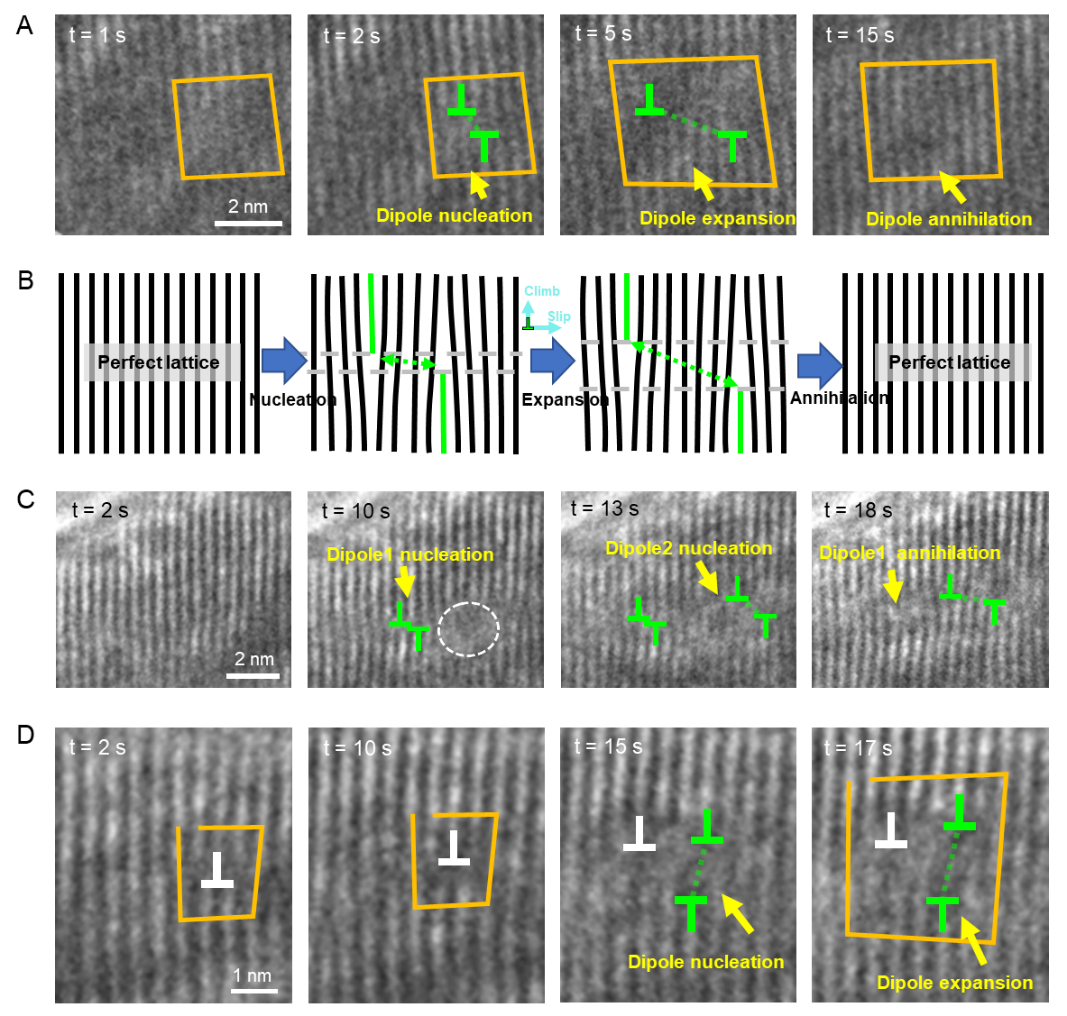
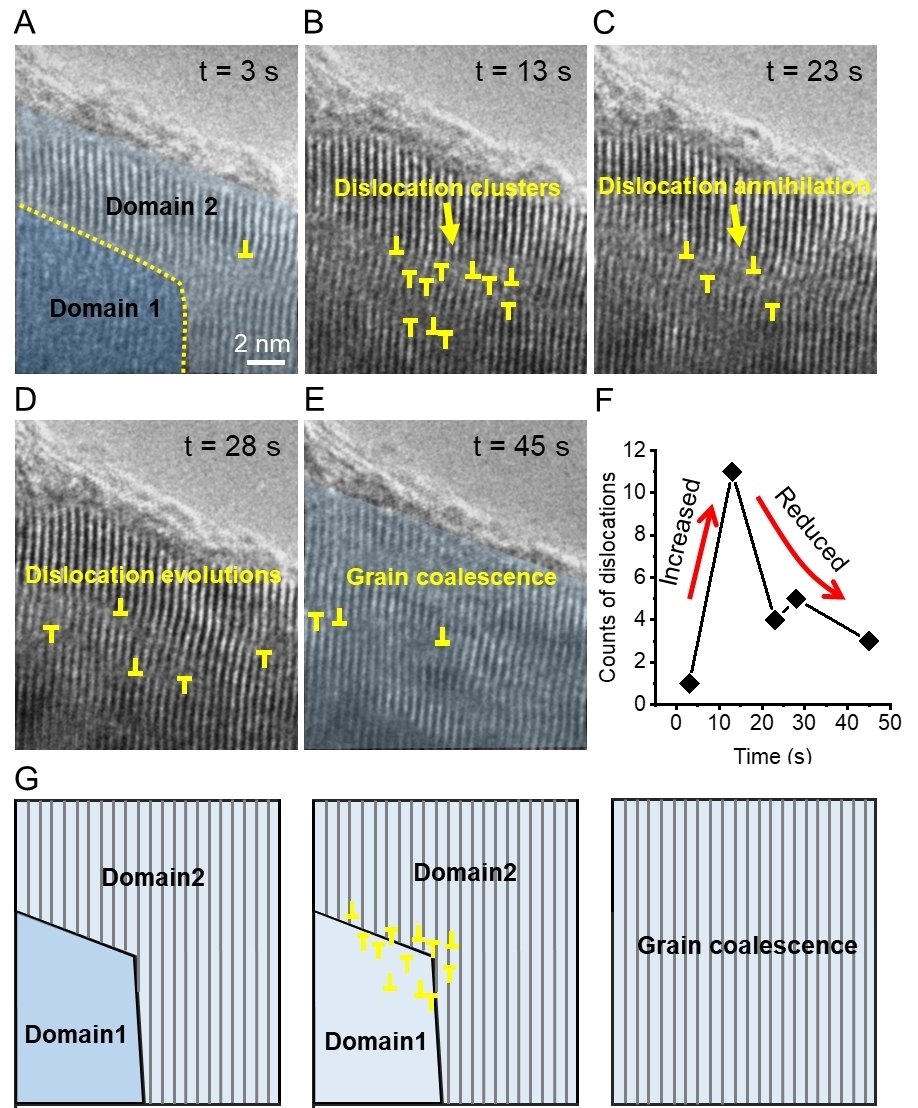
Dislocation nucleation, motion, and annihilation dynamics during electrochemical delithiation(Image by IMR)
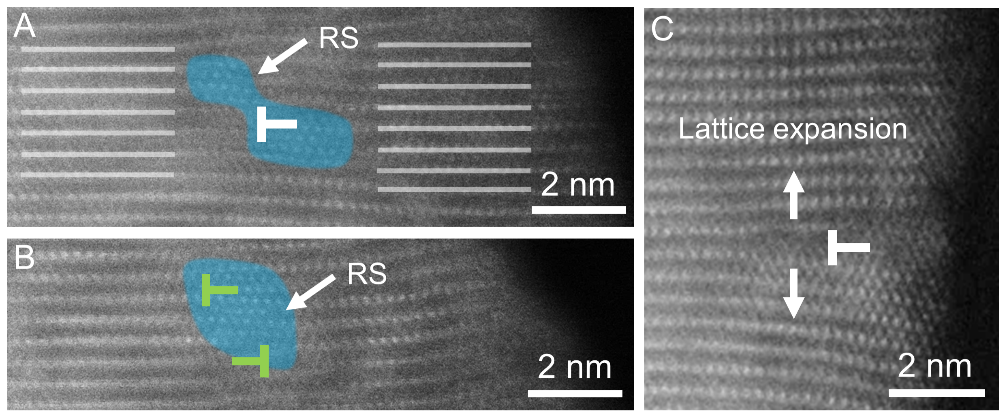
Dislocation-promoted concurrent cracking and rocksalt phase transformation (Image by IMR)
Dislocation burst-annihilation induced lattice reorientation(Image by IMR)