The world's first cardiovascular stent made of nickel-free high-nitrogen stainless steel developed by the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), has been approved for medical device registration by the National Medical Products Administration of China (NMPA Certificate No. 20253131417) in July, 2025.
This innovative cardiovascular stent, developed through collaboration of Zhongke Yi'an Medical Technology (Beijing) Co. Ltd. and IMR, addresses a significant clinical challenge: approximately 10% of the population exhibits nickel allergy, which increases the risk of restenosis after stent implantation, as reported in medical journals such as The Lancet.
Currently used main stent materials in clinic are 316L stainless steel and L605 cobalt-chromium alloy, which contain over 10% nickel, posing elevated risks for the nickel-sensitive patients.
To solve this severe clinical issue, the biomaterials research team led by Prof. YANG Ke developed a novel nickel-free high-nitrogen austenitic stainless steel (Fe-18Cr-15Mn-3Mo-1N) using the design strategy of "nitrogen replacing nickel". The new stainless steel containing over 0.8% nitrogen has demonstrated superior biocompatibility while achieving a high strength twice of 316L stainless steel and comparable to L605 alloy. Its exceptional mechanical safety and excellent hemocompatibility makes it ideal for vascular stent applications.
This novel stainless steel cardiovascular stent first received the CE Certification in January 2021, allowing to get into the international market. The approval of NMPA enables its clinical use in China, providing a safer option for the patients undergoing cardiovascular Interventional therapy, particularly for those with nickel allergies.
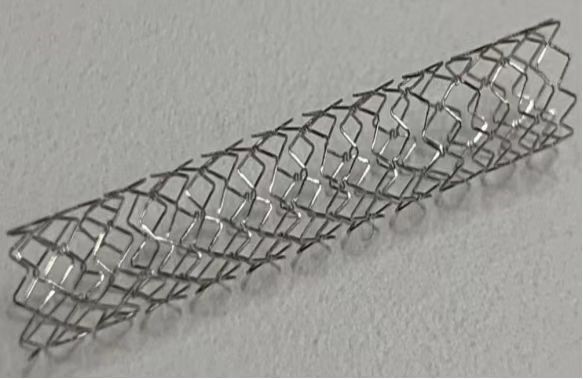
High-nitrogen nickel-free stainless steel cardiovascular stent (Image by IMR)