A research team from the Shenyang National Laboratory for Materials Science, the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), has developed a highly efficient and stable catalyst at the atomic scale for carbon monoxide (CO) oxidation. This breakthrough promises to advance environmental catalysis and the development of low-cost, high-performance catalysts.
The research, published as a cover article in the journal Nano-Micro Letters, addresses a long-standing challenge in catalysis. While single-atom catalysts maximize the use of precious metals, they often struggle to simultaneously activate multiple reactants like CO and oxygen (O₂). To overcome this, the team, led by Prof. LIU Hongyang and Prof. SUN Bo in collaboration with Chongqing University, constructed a catalyst with atomically dispersed platinum-ruthenium (Pt-Ru) pairs anchored on a defective graphene support.
Using a straightforward co-impregnation method, the team successfully created stable Pt-Ru dual-atom active sites. Advanced characterization techniques confirmed the formation of a direct Pt-Ru bond. Crucially, this bonding interaction enhanced the metallic nature of both atoms, enabling them to work in synergy. In the reaction, CO preferentially adsorbs to the Pt atom, while O₂ is effectively activated at the bridging site between the Pt and Ru atoms. This cooperative action overcomes the competitive adsorption limitation of single-atom catalysts and dramatically reduces the energy barrier for the reaction.
The resulting catalyst, designated Pt₁Ru₁/ND@G, exhibits exceptional performance. At a low temperature of 30 °C, it achieves a CO oxidation turnover frequency (TOF) of 17.6 × 10⁻² s⁻¹. This value is 10 times higher than that of a comparable platinum single-atom catalyst and surpasses the performance of most previously reported platinum-based catalysts. Moreover, the catalyst maintained stable activity during a 40-hour continuous test at 80 °C, demonstrating excellent durability.
This work not only provides an efficient synthesis strategy for dual-atom catalysts but also offers fundamental insights into their synergistic reaction mechanisms at the atomic level. It opens new avenues for designing next-generation, atomically precise catalysts for critical environmental and energy applications.

Catalyst structure characterization. (a-b), (d-f) HAADF-STEM images, (c) Linear intensity analysis, (g) Atomic distance statistics, (h) Ultraviolet–visible spectroscopy. (Image by IMR)
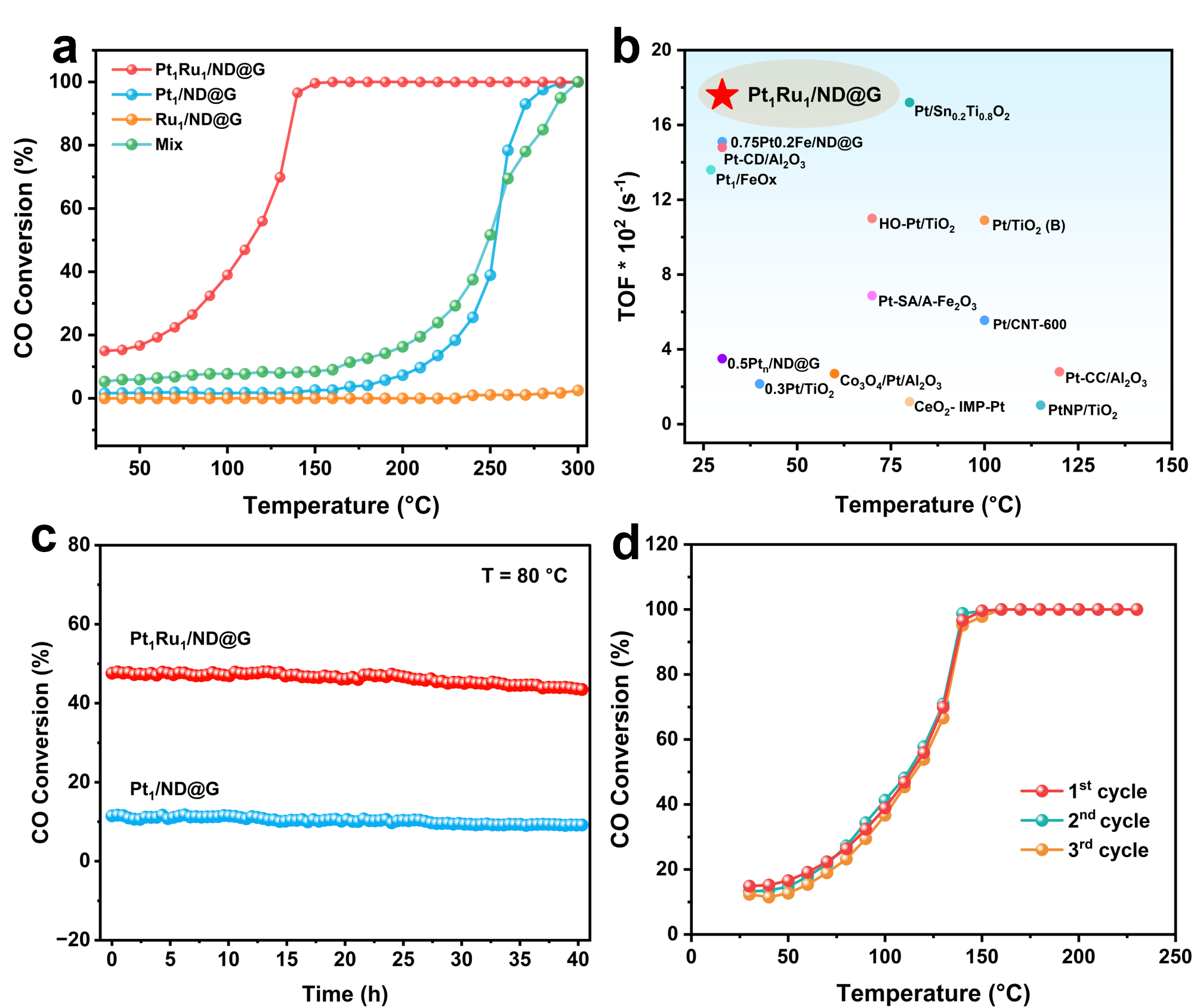
CO catalytic oxidation performance. (a) Reaction activity, (b) Performance comparison of Pt-based catalysts, (c) Long-term stability, (d) Cycling stability. (Image by IMR)
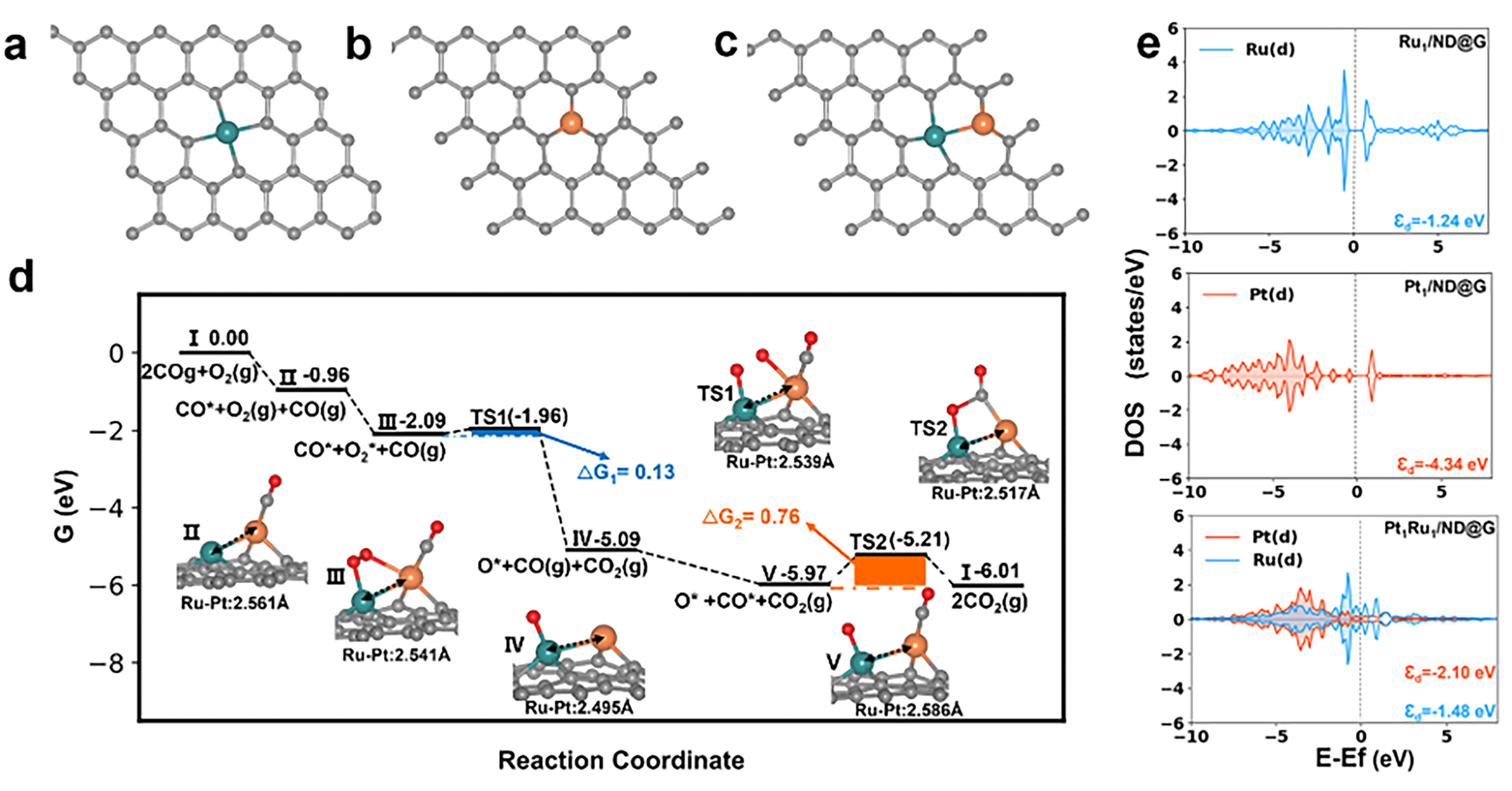
DFT theoretical calculations. (a-c) Catalyst structure models, (d) Reaction pathway for CO oxidation on Pt₁Ru₁/ND@G, (e) d-band center. (Image by IMR)
Schematic illustration of the CO oxidation reaction catalyzed by Pt₁Ru₁/ND@G. (Image by IMR)