A research team has developed a novel bond coat material that significantly enhances the oxidation resistance of thermal barrier coatings (TBCs) at 1200°C, a critical advancement for next-generation ultra-high-thrust aero-engines. The research, titled “Outstanding 1200 ℃ Oxidation Resistance in a Novel Multi-Principal Element Alloy via Lattice Distortion-Induced Diffusion Suppression,” has been published in Advanced Science.
Modern aviation engines, pushing for higher thrust-to-weight ratios and thermal efficiency, demand turbine inlet temperatures exceeding 1900°C, far beyond the melting points of superalloys. TBCs are thus essential, acting as a thermal insulating ceramic layer on turbine blades. The bond coat, situated between this ceramic topcoat and the superalloy substrate, plays a pivotal role. It must accommodate thermal stress and, critically, form a slow-growing, adherent thermally grown oxide (TGO) layer to protect the substrate from oxidation.
Since the 1970s, NiCoCrAlY alloys (MCrAlY) have been the standard bond coat material. However, they face a major limitation: oxidation rates skyrocket above 1100°C, leading to rapid TGO growth and spallation, which causes TBC failure. This temperature ceiling has persisted for decades, hindering engine progress.
To overcome this, a collaborative team from the Institute of Metal Research, Chinese Academy of Sciences (IMR, CAS), Peking University, and Shenyang University of Technology devised a dual-strategy focusing on both the initial and steady-state oxidation stages. First, they designed an alloy with a fine lamellar microstructure by optimizing eutectic aluminum content. This enhances aluminum supply during early oxidation, promoting the rapid formation of a continuous, protective α-Al2O3 scale. Second, they adjusted Co, Cr, and Ni ratios to maximize configurational entropy, creating a multi-principal element alloy (MPEA). The resulting severe lattice distortion in the aluminum-depletion zone beneath the TGO increases vacancy formation energy and raises the energy barrier for aluminum diffusion, effectively slowing down the oxidation process at high temperatures.
The newly developed NiCoCrAlYHf MPEA demonstrated exceptional performance. During 500-hour isothermal oxidation tests at 1200°C, its oxidation rate constant was only 1.28×10⁻¹² g²·cm⁻⁴·s⁻¹, approximately 59% lower than that of the traditional MCrAlY alloy. More importantly, in cyclic oxidation tests, the traditional alloy suffered oxide scale spallation after just 70 hours, with over 40% area loss after 500 hours. In stark contrast, the new alloy showed less than 2% spallation throughout the entire test, indicating superb scale adhesion and spallation resistance.
This breakthrough provides a crucial material foundation and a new design paradigm for developing bond coats capable of operating in more extreme environments, paving the way for future high-performance engines.
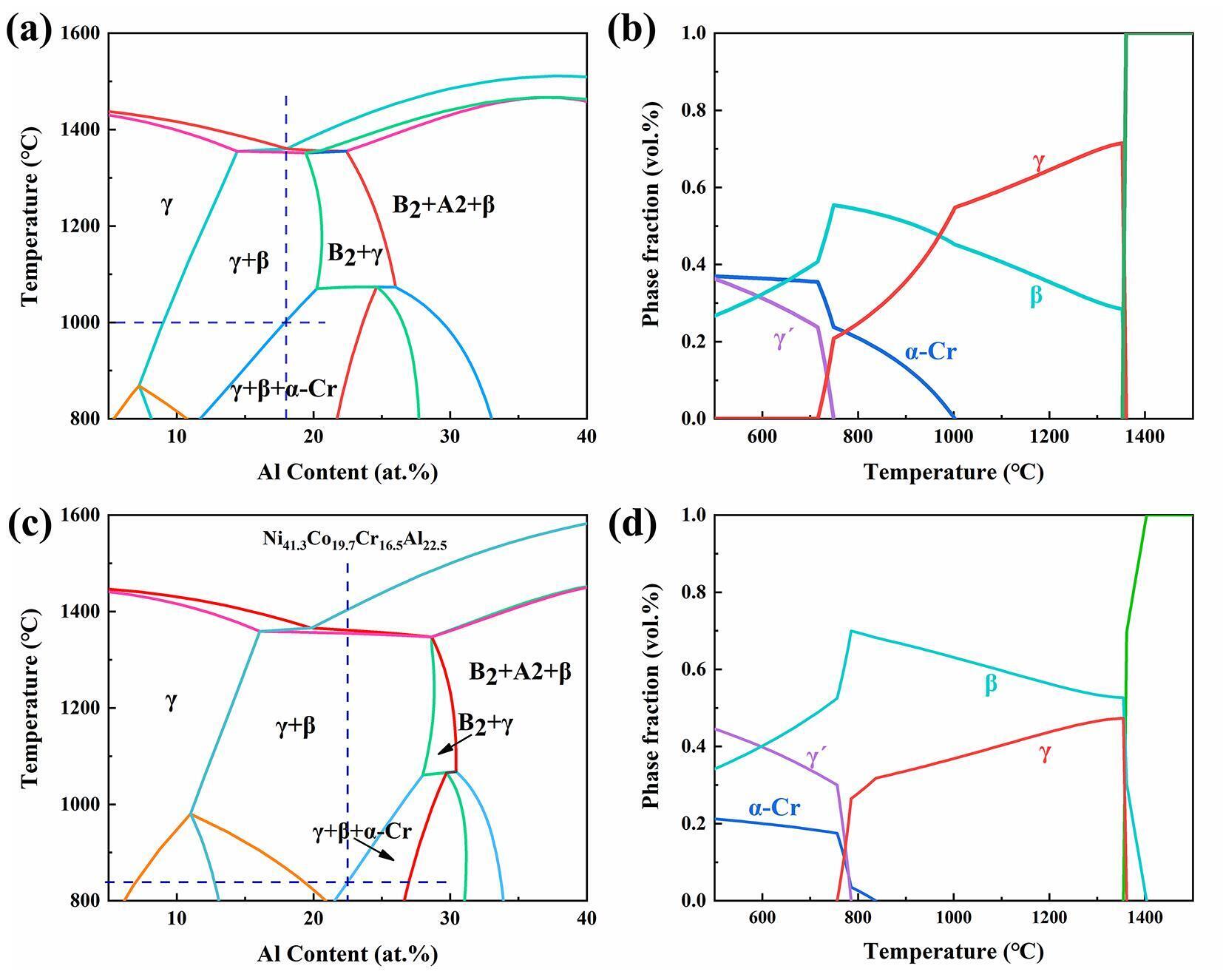
Design of eutectic multi-principal element alloys (a) Pseudo-binary phase diagram of the Ni50-xCo25Cr25Alx system; (b) Fraction of equilibrium phases as a function of temperature for the eutectic multi-principal element alloy Ni32Co25Cr25Al18; (c) Pseudo-binary phase diagram of the commercial MCrAlY alloy system Ni63.8-xCo19.7Cr16.5Alx; (d) Fraction of equilibrium phases as a function of temperature for the commercial MCrAlY alloy (Ni41.3Co19.7Cr16.5Al22.5). (Image by IMR)
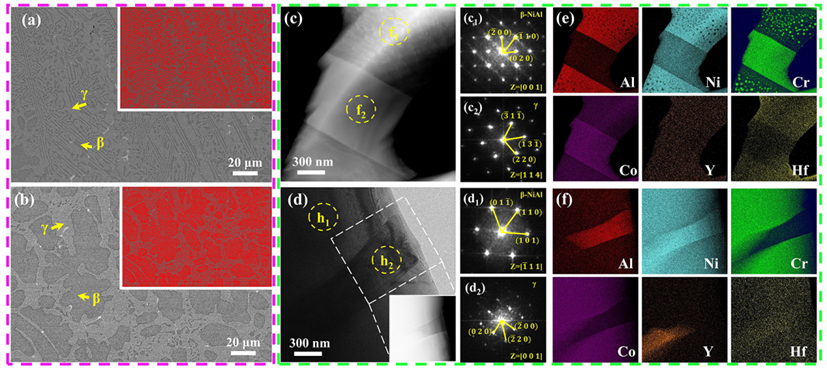
Microstructural characterization of the MPEA and commercial MCrAlY alloy (a, b) SEM images showing the lamellar microstructure in the MPEA, which is different from the typical ellipsoidal microstructure in the commercial MCrAlY; (c) TEM image of the MPEA; (c1, c2) FFT patterns of the regions marked f1 and f2 in (c); (d) TEM image of the MCrAlY; (h1, h2) FFT patterns of the regions marked h1 and h2 in (d); (e) STEM-EDS mapping of (c) showing the presence of Cr-rich nano-precipitates in the β phase; (f) STEM-EDS mapping of the inset region in (d) showing the absence of Cr-rich nano-precipitates in the β phase of the MCrAlY. (Image by IMR)
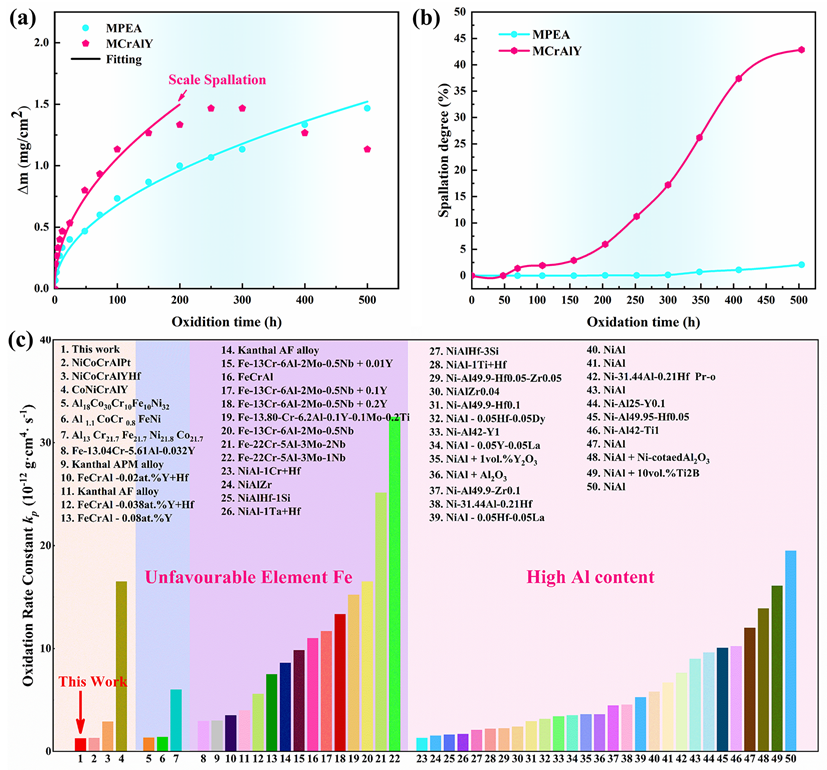
Oxidation resistance analysis of the MPEA and MCrAlY at 1200°C. (a) Isothermal oxidation kinetic curves of the MPEA and MCrAlY at 1200 °C; (b) Variation of the oxide spallation area fraction with time during cyclic oxidation at 1200 °C, indicating that the oxide film of the MPEA has better adhesion than that of the MCrAlY; (c) Comparison of the oxidation rate constant (kₚ) of the MPEA with that of existing Al₂O₃-forming alloys. (Image by IMR)

First-principles analysis of lattice distortion and Al diffusion rate in the ADZ of the two alloys. (a, b) Relaxed ADZ supercell structures of the MCrAlY (a) and MPEA (b); (c) Box plot showing the 1NN bond length distribution of different atomic pairs in the supercell structures; (d, e) HRTEM images of the ADZ of the two alloys; (f, g) Atomic-scale strain distribution maps of the HRTEM images obtained by the GPA method; (h) Energy barrier for Al diffusion in the ADZ calculated by the CI-NEB method; (i, j) Experimentally obtained Al concentration profiles from the ADZ to the Al2O3 film for the two alloys; (k, l) Al diffusion coefficients D(c*) in the ADZ of the two alloys calculated from the concentration curves in (i) and (j) using the Boltzmann-Matano method. (Image by IMR)